Chapter 55 Posterior Fossa Tumors in the Pediatric Population
Multidisciplinary Management
Primary brain tumors are the most common solid tumors in the pediatric population, comprising 20% to 25% of all childhood cancers. About 60% to 70% of all pediatric brain tumors originate in the posterior fossa.1–4 The reason that pediatric brain tumors have a propensity to occur in the posterior fossa has not yet been elucidated. By far, the most common posterior fossa tumors of childhood are medulloblastomas, ependymomas, and astrocytomas. As for uncommon tumors, these include atypical teratoid/rhabdoid tumors (AT/RTs), teratoma, hemangioblastoma, dermoid, and epidermoid. Each tumor may have a vastly different prognosis and response to treatment depending on the pathologies. Recent advancements have increased our knowledge of the cell biology, facilitated earlier diagnosis and improved neurosurgical resections and adjuvant treatment. These in turn have not only improved the survival of children but also their quality of life. Although much progress has been made in the diagnosis and treatment of posterior fossa tumors, they still cause the most cancer-related deaths in this age group.5,6 This review focuses on the multidisciplinary management of the major types of pediatric posterior fossa tumors. Current advances in tumor diagnosis and surgery, along with adjuvant therapeutic modalities, will be discussed.
Symptoms and Signs
Posterior fossa tumors often cause hydrocephalus by blocking cerebrospinal (CSF) outflow pathways, with resulting signs and symptoms of raised intracranial pressure (ICP).7 Symptoms typically begin with intermittent headache, often worse in the morning followed by vomiting and eventually gait disturbance. Funduscopy must be performed in children with suspected posterior fossa or supratentorial tumors because papilledema is a common finding. Sixth nerve palsies resulting from intracranial hypertension and ataxia from a combination of cerebellar compression and hydrocephalus are also common.
The presenting signs and symptoms are dependent on the growth rate of tumors, the age of the patient and the location of the tumor. Most children with medulloblastomas present with a short clinical history, for less than 1.5 months in approximately 50% of patients and less than 3 months in approximately 75% of patients.7 Ependymomas are relatively fast-growing tumors and the median duration of symptoms is 2 to 3 months.8 As for cerebellar astrocytoma, the symptomatic period ranges from 5 to 9 months and is usually significantly longer than other posterior fossa tumors.9 Rapid, sudden deterioration in neurologic status may result from acute obstructive hydrocephalus or hemorrhage into either the tumor or the subarachnoid space.10–13 Older children may complain of headache, neck stiffness, dizziness or diplopia. On neurologic examination, they will demonstrate truncal or appendicular ataxia, dysmetria, nystagmus, or cranial nerve palsies. In comparison with older children, the presentation in infants and young children is often more insidious, with gradual progression.14 The diagnosis is suspected in the setting of irritability, loss of appetite, weight loss and failure to thrive. In addition, they may display signs of increased ICP, including lethargy, drowsiness, vomiting, sun-setting, a full fontanelle, or an increasing head circumference. Midline tumors of the cerebellum usually cause truncal ataxia, whereas hemispheric lesions cause ipsilateral dysmetria. Tumor in the obex and area postrema causes vomiting even if the tumor is small and there is no hydrocephalus. Cranial neuropathies such as facial weakness, hearing loss and swallowing dysfunction may present in tumors that exit the foramen of Luschka from the cerebellopontine angle or in tumors demonstrating brain stem invasion.
Head-tilt, signifying descent of the cerebellar tonsils into the foramen magnum with compression of the C1 or C2 nerve roots, may be observed. Development of a stiff neck or head tilt usually suggests tonsillar involvement by the tumor or signs of impending herniation.15–17 Rarely, children initially present with symptoms of spinal dissemination, such as back and leg pain or paraparesis.18 The transmission of increased pressure to the upper cervical spinal cord may cause syringomyelia and related symptoms in patients with cerebellar astrocytomas.19
Radiologic Findings
Medulloblastoma
Approximately two thirds of medulloblastomas are located in the midline arising from the vermis or inferior medullary velum and widening the space between the cerebellar tonsils. Isolated involvement of the hemisphere is seen in older children and young adults.20,21
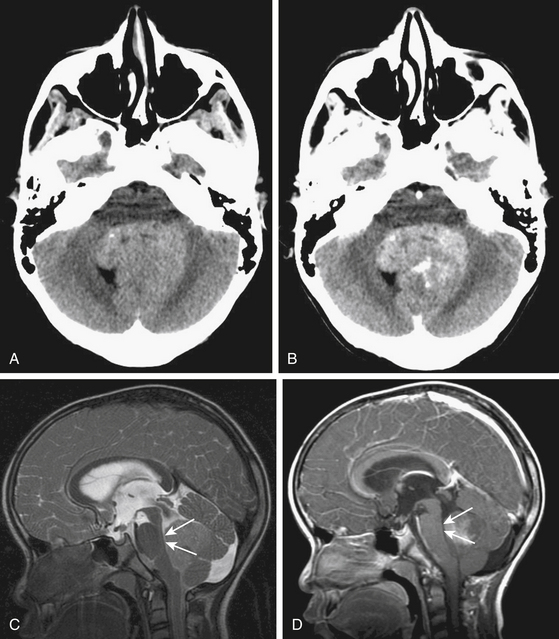
Due to the increased nuclear to cytoplasmic ratio of the tumor cells, medulloblastoma typically appears as a homogenous iso- to hyper-dense midline mass within the posterior fossa on non-contrast CT scan (Fig. 55-1A), with variable amounts of peritumoral edema and hydrocephalus whereas the solid portion of cerebellar astrocytomas are hypodense on precontrast studies.22 By CT, medulloblastoma can also contain calcification, necrosis, cystic degeneration, and hemorrhage and can invade the brain stem. Following contrast administration, this tumor demonstrates homogenous enhancement (Fig. 55-1B).
On MRI, medulloblastoma typically appears as a hypo- to iso-intense mass on T1-weighted images (T1WI), and iso- to hyper-intense on T2-weighted images (T2WI)22,23 (Fig. 55-1C). Diffusion-weighted images (DWI) demonstrate restricted diffusion.24 The enhancement pattern is variable: enhancement may be uniform or patchy22,23 (Fig. 55-1D). The heterogeneity probably results from cysts and calcification. Intracranial subarachnoid or intraventricular dissemination should be carefully identified because the perimesencephalic cisterns, tentorial surface and suprasellar cisterns are frequent sites for dissemination at the time of tumor diagnosis.
Ependymoma
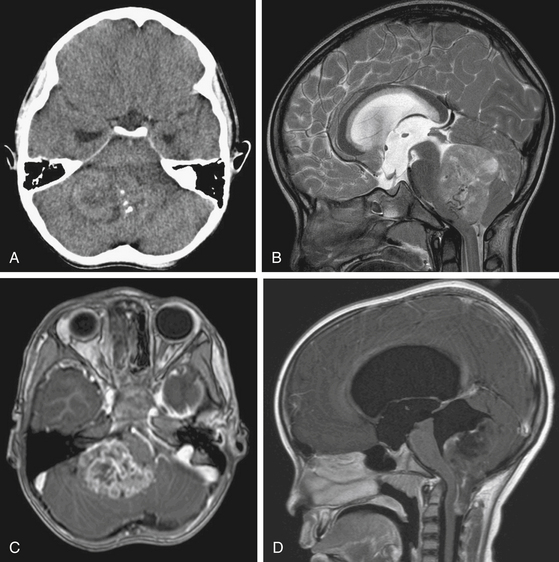
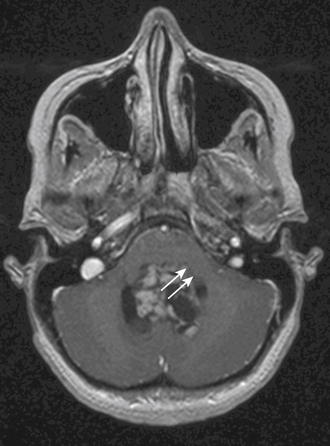
On CT scan, a posterior fossa ependymoma is an iso- or hyper-dense midline cerebellar mass with calcifications, small cysts, and heterogeneous enhancement after intravenous contrast administration25 (Fig. 55-2A). Hemorrhage is present in up to 13% and calcifications are frequent in 25% to 50%. MR imaging shows marked heterogeneity of the tumor due to small cysts as well as areas of old hemorrhage. Precontrast T1- and T2-weighted images commonly reveal an iso- to hyper-intense signal intensity. Most tumors show heterogeneous and irregular enhancement following gadolinium administration26 (Fig. 55-2B).
The most characteristic appearance supporting the diagnosis of ependymoma is of a mass arising from the lower brain stem and projecting into the fourth ventricle; in half or more tumors, extension through the foramen of Luschka into the cerebellopontine angle or through the foramen magnum into the cervical spinal canal is found (Fig. 55-2C and D). The vertebral arteries and posterior inferior cerebellar arteries may be displaced or encased.
Cerebellar Astrocytoma
Cerebellar astrocytomas can be either vermian or hemispheric in location. They can be predominantly cystic with a solid mass, the so-called mural nodule, in 30% of cases; they can be, solid with cystic areas in 40% to 50%; and wholly solid in 20% to 30%.9,27,28 Cystic tumors tend to be located within the hemispheres and solid tumors in the midline alone or in combination with extension into one or both hemispheres.29,30
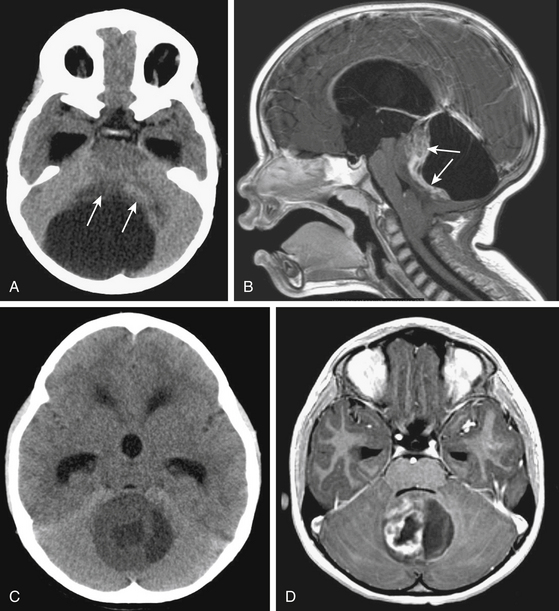
On noncontrast CT, the solid portion of the tumor is usually iso- to hypodense and the cyst is hypodense but denser than CSF because of the high protein concentration (Fig. 55-3A and C). Calcification can be seen in up to 20%, and cysts in 70%.31,32 On MRI, solid parts of the tumor are generally iso- or hypo-intense signal intensity masses on T1-weighted sequences and hyperintense masses on T2-weighted and FLAIR sequences.33 Contrast enhancement is usually irregular caused by cysts and tumor necrosis22 (Fig. 55-3B and D). Tumors of pilocytic pathology show intense enhancement of their solid portions.34 Nonenhancing tumors are almost never of pilocytic histology. Frequently, the infiltrative fibrillary astrocytomas do not show much gadolinium enhancement.35 Both pathologies can invade the cerebellar peduncle and brain stem.
Atypical Teratoid/Rhabdoid Tumors
AT/RTs usually originate in the cerebellar hemispheres instead of the midline and have a predilection for the cerebello-pontine angle.36,37 Their pattern of growth is aggressive with significant mass effect on the adjacent fourth ventricle and brain stem, and early CSF dissemination. AT/RTs may also arise in the spinal cord, pineal gland and suprasellar region.38,39
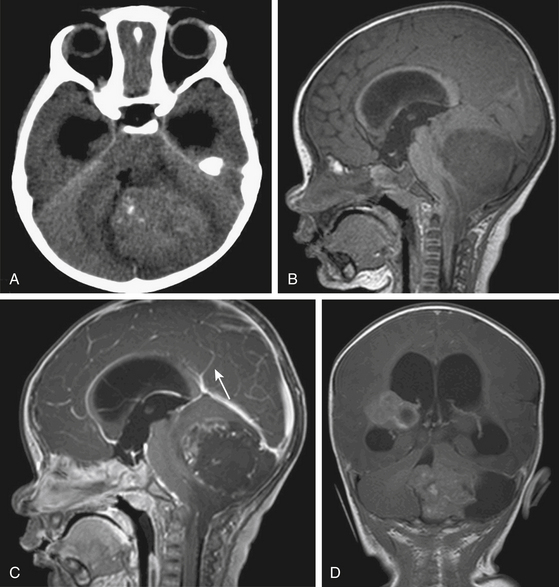
On noncontrast CT, the solid portions of the tumor demonstrate hyperdensity, presumably due to the high cellularity of the tumor36,38 (Fig. 55-4A). On MRI, solid portions of the tumor are low signal intensity on T1-weighted images and isointense or decreased signal on T2-weighted images.36,38,40 In addition, frequent necrosis, cysts, and sometimes hemorrhage are identified, giving an appearance of striking heterogeneity.36,37,41 Enhancement can be variable (Fig. 55-4B to D).
Invasion of the Brain Stem and Cerebellar Peduncle
Invasion of the brain stem and cerebellar peduncle is found in up to 40% of newly diagnosed medulloblastoma.4,42 In another study, brain stem or peduncle involvement is identified in 34% of cerebellar pilocytic and diffuse astrocytomas.43 Cerebellar peduncle involvement is suggested by an ill-defined border on the affected side on postcontrast MRI (Fig. 55-5). On MR midsagittal images without/with contrast infusion, a cerebrospinal fluid space between the tumor and the floor of the fourth ventricle may be found, which can indicate that there is no invasion of the floor of the fourth ventricle (Fig. 55-1C and D). However, absence of a cerebrospinal fluid space does not always indicate the invasion of tumor. As such, it is difficult to affirm unequivocally that the tumor is compressing or invading the floor of the fourth ventricle by imaging studies alone.
Work-Up for Tumor Dissemination
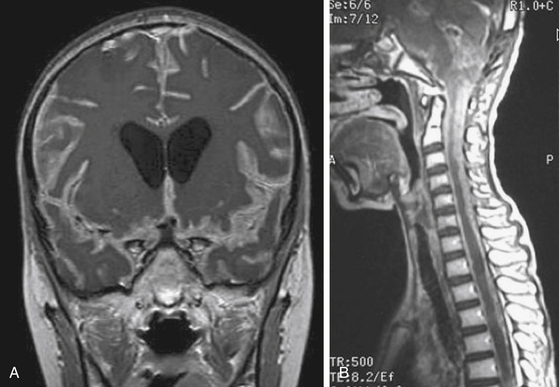
At diagnosis, patients rarely present with symptoms of tumor dissemination. However, spinal metastases can be found in about 20% to 50% of medulloblastoma, 11% to 17% of ependymoma, 25% to 34% of AT/RT at the time of diagnosis.38,39,44–49 The current standard of care should be to obtain pre- and post-contrast scans of the brain and spine when the presumptive diagnosis of a malignant posterior fossa tumor is made. In the first few weeks after posterior fossa surgery, artifacts from blood products can be very difficult to differentiate from CSF spread of tumor.50,51 These problems are avoided by performing preoperative staging MR studies of the spine. Postsurgical MRI should be performed within 48 to 72 hours of surgery or at least 2 weeks later to avoid the difficulty of distinguishing between postoperative changes and tumor dissemination or residual tumor. By MRI scans, leptomeningeal dissemination appears as diffuse enhancement of the meninges and/or enhancing clumps along the spinal cord and cauda equina on T1-weighted images (Fig. 55-6).
In addition to spinal MRI, CSF cytologic examination for malignant cells has been used for the diagnosis of leptomeningeal disease pre- or post-operatively. However, spinal MR imaging has a greater diagnostic accuracy than CSF cytology in the early detection of disseminated disease.52,53 CSF cytologic analysis performed more than 2 to 3 weeks after surgery can reduce false-positive results. Negative CSF cytologic results do not always exclude the tumor dissemination.54,55
Surgery
Management of Hydrocephalus
Obstructive hydrocephalus is reported in 70% to 80% of children with posterior fossa tumors and is frequently the cause of clinical deterioration at the time of diagnosis.56,57 Depending on the patient’s symptoms and the severity of the associated hydrocephalus, a decision must be made whether to treat the hydrocephalus up front or at the time of tumor resection. In the 1960s, when a child presented in a poor clinical state as a result of a delayed diagnosis, the routine placement of a preoperative shunt significantly reduced the overall morbidity and mortality rate.58,59 However, neurosurgeons have questioned the need for a routine shunt because only 15% to 30% of patients ultimately require permanent CSF diversion following resection of the tumor.57,60 In addition to the usual complications associated with ventriculoperitoneal (VP) shunts such as infection and blockage, rare complications such as upward herniation, tumor hemorrhage, and peritoneal seeding of the intracranial tumor may occur.61 Accordingly, most centers are using a combination of corticosteroids, early surgery, and external ventricular drainage rather than VP shunting.62,63
Postoperative hydrocephalus occurs within the first few days to months after surgery. Rarely, it will develop several months or years after surgery, or at the time of a local recurrence or of subarachnoid spread.64,65 Factors requiring placement of postoperative shunt include more severe hydrocephalus at diagnosis, young age, large preoperative tumor size, midline localization and incomplete tumor removal.56,60,66,67 Patients with medulloblastoma, as opposed to other posterior fossa tumors, also appear to be at higher risk for postoperative hydrocephalus requiring a VP shunt.56,68
The effectiveness of endoscopic third ventriculostomy (ETV) has been evaluated in the management of preoperative and postoperative hydrocephalus. ETV, when performed prior to posterior fossa surgery, reduces the incidence of hydrocephalus because preoperative normalization of CSF hydrodynamics decreases the risk of permanent postoperative impairment of the CSF circulation.67 However, the routine application of preoperative ETV is not indicated because of the small number of patients requiring definitive treatment for hydrocephalus.57,68 ETV may be used for persistent or progressive hydrocephalus following tumor removal.57
Patient Positioning
Several options for patient positioning are available for patients with posterior fossa tumors: prone, Concorde, lateral decubitus, “park-bench” and the sitting position. Pediatric patients are typically placed in the prone position. For lesions extending superiorly through the aqueduct, the head is tilted slightly to the patient’s right, away from the surgeon and flexed far forward (Concorde position) so that the rostral extent of the tumor can be removed with the surgeon sitting behind the patient’s shoulder. The lateral decubitus positioning can be used for tumors that occur in the cerebellopontine angle or lateral cerebellum. The sitting position has several distinct advantages including limited use of retraction, and a clear operative field. However, it is difficult to position infants and young children in the sitting position. Dangers include cardiovascular instability, hypotension, air embolism, pneumocephalus, subdural hematoma, and the rapid escape of cerebrospinal fluid from the ventricular system.
Opening and Closure
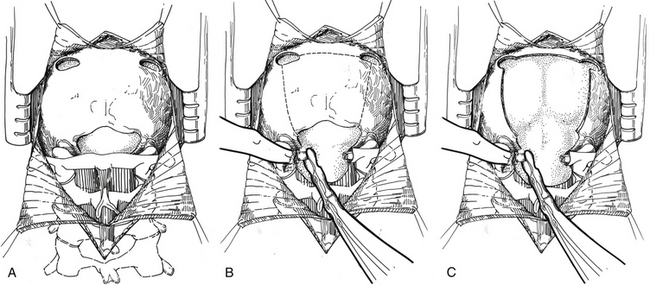
If the lesion is located in the vermis or paravermian region, a midline skin incision suffices. If the lesion occupies one hemisphere without a contiguous attachment to the midline, a paramedian incision is preferred and a hockey stick incision can be used to allow for a wider craniectomy. In the midline posterior fossa exposure, the incision is extended from slightly rostral to the inion down to the C1–C2 spinal process. Skin flaps are mobilized by undermining the subcutaneous plane to prepare a fascial flap for closure and the fascia is incised using a Y- or T-shaped incision for reapproximating tightly at the superior nuchal line. The cervical musculature is mobilized laterally off the occiput and cervical laminae in the midline avascular plane. The foramen magnum is exposed, and the dura is identified up to the posterior arch of C1. A posterior fossa craniotomy is then performed (Fig. 55-7). Bilateral burr holes are made just below the transverse sinus from the midline. The dura is separated from the inner table of the skull, which is typically not adherent in children. At the foramen magnum, the junction of the pericranium and the dura is sharply dissected with caution because of the occipital sinus in the midline near the foramen magnum. If the tumor extends into the upper cervical spinal canal, a laminectomy of the upper vertebrae may be performed. Monopolar cautery should be used carefully when dissecting the superolateral surface of C1 to prevent injury to an aberrant vertebral artery. C1 can be bifid and is often cartilaginous in infants and young children. After extending a laminectomy below C2, young children have an increased risk of swan neck deformity.69 Therefore, it is prudent to remove the smallest amount of bone possible.
The dura is then opened in a Y-fashion with care taken to obtain hemostasis of the occipital sinuses (Fig. 55-8A). When the vertical incision extends to the foramen magnum, it may extend below the falx cerebella. If there is significant bleeding from the midline occipital sinus and circular dural sinuses, it should be controlled with obliquely placed hemostatic clips or suture ligatures. If clips are used, they can be removed as the dura is sutured because metal clips may result in artifact on postoperative MRI studies. Intraoperative ultrasonography and neuronavigation may be used to define the location of tumors. The surgeon should inspect the surface of the exposed cerebellum and spinal cord for evidence of leptomeningeal seeding. After the cisterna magna has been exposed, cerebrospinal fluid is aspirated for cytology and drained to decrease ICP.
After tumor removal, meticulous hemostasis is achieved with judicious use of bipolar coagulation and gentle pressure, and may be confirmed with a Valsalva maneuver. The dura is always closed in a watertight fashion, followed by replacement of the bone flap and multilayer closure of the superficial tissues. It is important to close the fascial layer tightly near the inion to prevent postoperative leak of cerebrospinal fluid and the formation of a pseudomeningocele.
Tumor Removal
Transvermian and Telovelar Approaches to Midline Cerebellar Tumors
The two most common surgical approaches to midline cerebellar tumors adjacent to the fourth ventricle are the transvermian and telovelar approaches. The transvermian approach involves incising the inferior vermis of the cerebellum and retracting the two halves of the vermis in opposite lateral directions to remove the tumor. The telovelar approach uses the dissection of the cerebellomedullary fissure, which is a natural cleft between the cerebellum and medulla oblongata, and the dissection of the tela choroidea and inferior medullary velum along the natural avascular planes.70–74 The greatest advantage of the telovelar approach is the superolateral exposure of the fourth ventricle and the complete exposure of the foramen of Luschka, which can be accessed without removal of cerebellum or adjustment of the retractors.75,76 However, a limitation of this exposure can be expected when approaching the rostral portion of the fourth ventricle. The transvermian approach provides a greater working angle in this area and a better visualization of the midline inferior portion of the superior medullary velum and fastigium. Nevertheless, the main disadvantages of this approach are a limited lateral exposure and the potential for complications due to iatrogenic injury from excessive vermian dissection and retraction.
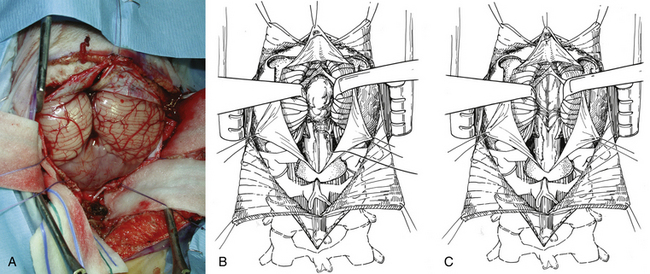
In the transvermian approach, the cerebellar tonsils are separated in the midline and it is sometimes helpful to retract the inferior vermis rostrally or incise the caudal vermis to prevent injury to the inferior vermian veins. The posterior inferior cerebellar arteries are usually displaced laterally. The distal portion of the vermis is divided to expose the dorsal surface of tumor (Fig. 55-8B). Cottonoid patties are used to help to protect the normal neural structures and to dissect along the tumor–cerebellar interface. Once planes around the tumor are developed, the tumor is internally debulked, with suction or with the Cavitron. Specimens are now taken for pathologic assessment and tumor banking. As the tumor is debulked, the lateral tumor–cerebellum interface continues to be developed. The uninvolved side of the cerebellar peduncle is first dissected from the surrounding cerebellar tissue, the floor of the fourth ventricle and the aqueduct are then identified, and tumor invading the involved cerebellar peduncle is resected (Fig. 55-8C). The dentate nucleus is located just above the ipsilateral tonsil, and one should take meticulous care to avoid trauma to it during resection of the tumor from the upper pole of the tonsil.
The telovelar approach is performed by opening the cerebellomedullary fissure, which includes dissection of the tonsillomedullary and uvulotonsillar space. The two cerebellar tonsils are then retracted laterally to expose the floor of the fissure, which includes the inferior medullary velum and tela choroidea. The tela choroidea, which forms the caudal part of the roof of the fourth ventricle, is incised from the foramen of Magendie and then followed laterally to the foramen of Luschka on both sides. In large tumors, the uvulotonsillar cleft is stretched, and the anatomy may be distorted. Aggressive dissection in this area before decompression can result in breaching the pial plane and entering either the vermis or tonsil leading to neurologic deficit, such as ataxia and cerebellar mutism. Placing the retractor on the superomedial part of the tonsil to visualize the superolateral corner of the fourth ventricle can injure the dentate nucleus. Dissection and decompression should be done simultaneously to minimize retraction and reduce associated injuries in large-sized tumors.77,78
Techniques When Operating Near the Brain Stem
Care must be taken to identify areas where tumor may invade the floor of the fourth ventricle. One of the most important factors of successful tumor resection is the identification of the floor of the fourth ventricle. If the tumor is not adherent to the floor of the fourth ventricle, one can place Cottonoid patties along the ventricular floor as the dissection proceeds. If the tumor adheres to or invades the floor of the fourth ventricle, the intact floor of the fourth ventricle next to the invasive tumor is identified and the last portion of the tumor is resected at the plane of the floor of the fourth ventricle. The sylvian aqueduct should be covered with Cottonoid patties to prevent blood from entering the third ventricle. The tumor should not be chased into the brain stem as this will lead to serious neurologic morbidity. Hemorrhage from the floor of the fourth ventricle should not be cauterized, because this may result in unexpected cranial nerve damage. Placement of a Gelfoam pack and gentle compression should result in the hemostasis needed.
Intraoperative Neuromonitoring
Intraoperative monitoring of the cranial nerves and the brain stem is frequently used for posterior fossa tumors. Monitoring may be helpful to decrease morbidity and to allow a more aggressive resection. Brain stem auditory-evoked potentials (BAEPs) are resistant to most anesthetic agents. Somatosensory-evoked potentials (SSEPs) are affected by the inhalation anesthetics, although the effects to nitrous oxide are minimal.79 Intravenous anesthetics affect SSEPs significantly less than do inhalation anesthetics. Propofol with nitrous oxide has minimal latency shift and amplitude reduction effects on SSEPs and offers promise as an effective adjunct to neuroanesthesia.80,81
Operative Complications
The most common complication is cerebellar ataxia. This may be transient and often rapidly resolves over several weeks. The portion of the tumor involving the unilateral cerebellar peduncle can be aggressively resected, and gross total resection is possible with minimal neurologic deficits.82 However, if bilateral cerebellar peduncles are involved, radical surgical manipulation should be avoided and may result in severe permanent cerebellar ataxia. When the dentate nucleus is damaged, the disequilibrium and intentional tremor during voluntary movement of the extremities may be found.
Cerebellar mutism is a unique postoperative complication seen in 5% to 30% of children following the resection of a posterior fossa mass lesion.71,83,84 Vermian incision and lateral retraction of the dentate nuclei may explain the development of the cerebellar mutism syndrome particularly in children. Cerebellar mutism is a transient complication that may appear after removal of midline cerebellar tumors involving the vermis in children. It typically arises 1 to 2 days following surgery with patients initially displaying diminished speech progressing to mutism and typically resolves during the ensuing weeks to months. Cerebellar mutism is characterized by a lack of speech output in awake patients with intact speech comprehension, and is sometimes associated with oropharyngeal apraxia.
Cranial nerve dysfunction, such as abducens palsy and facial weakness, internuclear ophthalmoplegia, horizontal gaze palsy, swallowing difficulty, and vocal cord palsy are related to irritation of the brain stem or lower cranial nerve. Deficits with vocal cord apposition and swallowing may cause complications such as aspiration pneumonia and respiratory distress. Swallowing problems related to lower cranial nerve manipulation can generally be expected to recover function over time.3,85,86 On the contrary, the symptoms with nuclear involvement of the brain stem may take longer to recover or may never recover completely.
Postoperative pseudomeningoceles or subsequent CSF leak affect 23% to 28% of all children with posterior fossa tumors.87,88 The mechanisms leading to pseudomeningocele formation are unclear, but probably include inadequate dural and wound closures. Small pseudomeningoceles may respond well to pressure bandages, needle aspiration, and lumbar CSF drainage. However, pseudomeningoceles may be a manifestation of hydrocephalus and in some cases may require CSF diversion to control.
Specific Tumors
Medulloblastoma
Epidemiology and Genetics
Medulloblastomas are the most common malignant brain tumor in children comprising 20% to 25% of all pediatric brain tumors and are usually found in the posterior fossa.89 The majority (85%) arise from the cerebellar vermis and in the minority, they arise laterally from the cerebellar hemisphere. The median age at diagnosis is approximately 6 to 9 years.20,90 There is a slight male predominance (male:female ratio 2:1).91,92 Up to 30% of cases occur in adulthood.93
Several cancer predisposition syndromes are associated with medulloblastoma including Gorlin’s syndrome, Turcot’s syndrome, Li-Fraumeni syndrome, and Rubenstein-Taybi syndrome.94 A frequent genetic abnormality is amplification of the MYC family of oncogenes, which is seen in up to 10% of cases.95–97 Molecular subgroups, characterized by Wnt/wingless signaling, sonic hedgehog signaling (SHH), expression of neuronal differentiation genes or photoreceptor genes have been identified.97–99 Based on these signaling pathways, targeted therapies have been suggested using agents such as cyclopamine and HhAnTag (small molecule inhibitor of the SHH).100–102 Common chromosomal copy number changes include gain of chromosomes 1q and 7, as well as loss of chromosomes 22, 11, 10q, and 17p.95,103,104 Loss of 17p is observed in up to 50% of cases, and may occur in the context of an isochromosome 17q.95,103
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree