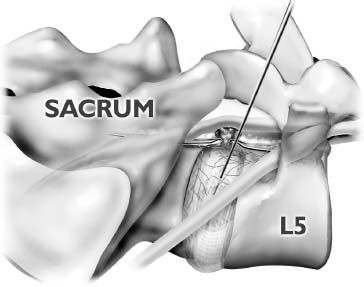
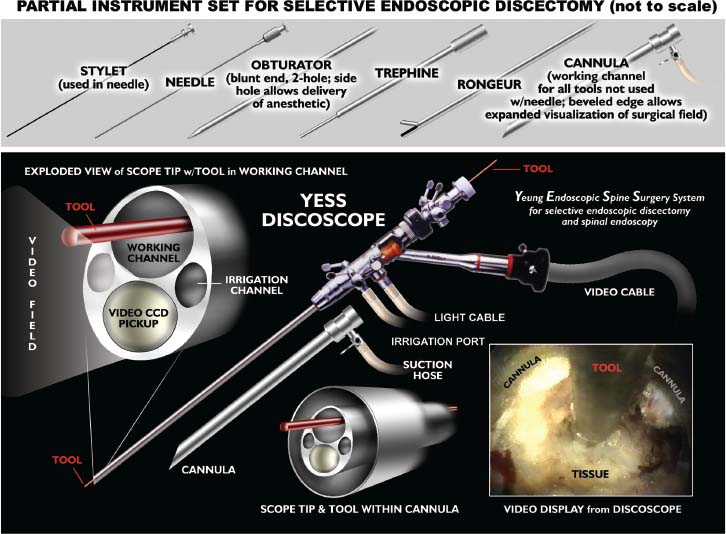
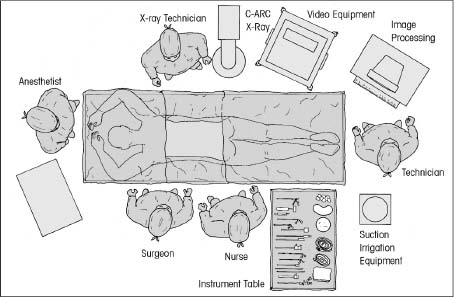
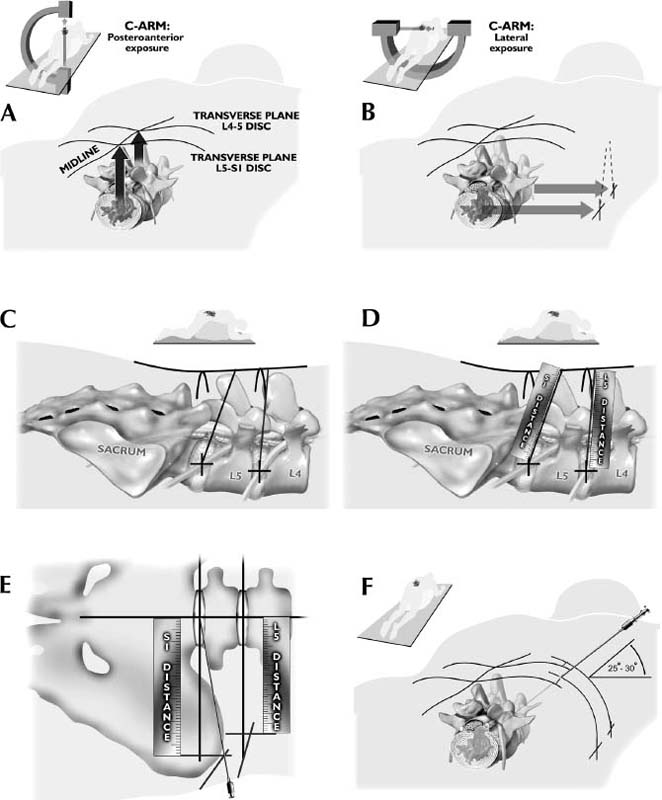
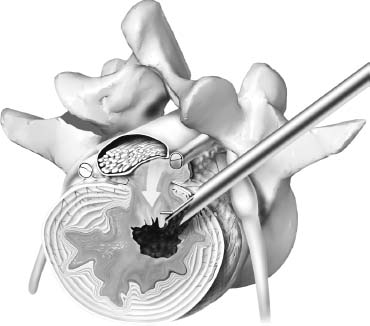
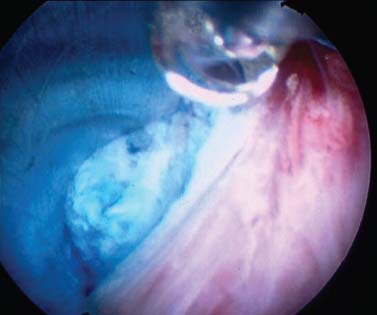
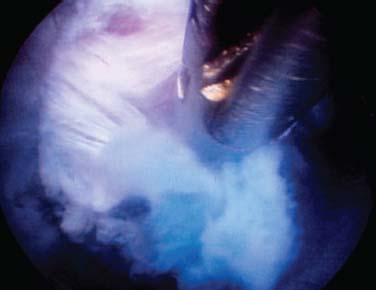
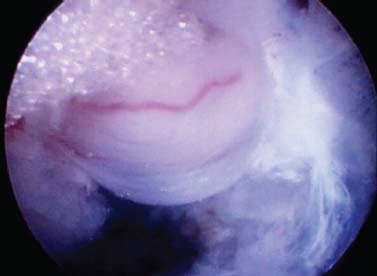
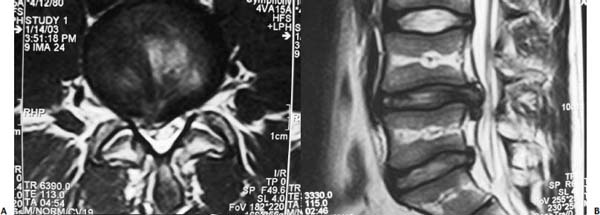
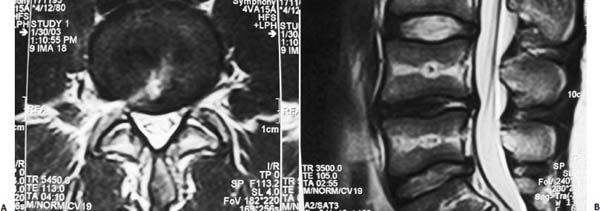
The intervertebral disk, an important supporting structure of the spinal column, is implicated as a major source of low back pain and sciatica.1 The pathogenesis of disk degeneration and herniation is complex and multifactorial, but it was clearly outlined and documented by Wolfgang Rauschning’s work illustrating the pathoanatomy of degenerative disk disease and degenerative conditions of the lumbar spine.2 Most disk herniations are not the result of an acute event; rather, they are an accumulation of several insults to the spine that lead to degeneration, annular tears, and eventual disk herniation. There are several theories of disk degeneration, including mechanical, chemical, age-related, autoimmune, and genetic. Within the mechanical theory, the following types of abnormal loads have been proven experimentally to cause disk injury: torsion, compression, repetitive compressive loading in flexion,3 hyperflexion,4 and vibration. Disk surgery has traditionally been reserved for disk herniations causing radiculopathy or nerve deficits due to mechanical compression on the spinal nerves. This is due to the inherent morbidity of the posterior surgical approach that must violate and alter the important function of the posterior spinal column. Open posterior diskectomy often includes or requires a midline incision, muscle and ligament stripping, prolonged muscle retraction, bone resection of the lamina and facet, and nerve root and dural tube retraction. This can cause instability and scarring around the sensitive nerve roots, even in a technically perfect operation. The morbidity of the standard posterior approach has therefore limited the use of surgery as an early treatment option in the cascade of disk degeneration and herniation. Thus, surgery was often not recommended for herniations without neurologic deficits, “small” herniations, central herniations, and annular tears. The dogma that “disk surgery is really decompressive nerve surgery” dominates the rationale for traditional microdiskectomy for herniated disks. Minimally invasive surgical options that limit the inherent approach-related morbidity are possible with the posterolateral portal.5–13 This approach to the disk is most challenging at the L5–S1 level due to the prominence of the iliac crest. Most L5–S1 disk spaces are accessible; however, entry into the disk may require foraminal decompression of the lateral facet. The least invasive of all posterolateral intradiskal techniques is the injection of chymopapain, a treatment option validated by at least two large prospective, randomized double-blind studies and numerous cohort studies.14 This treatment produced satisfactory results in many studies and came into widespread clinical use in the 1970s, but it lost popularity with reports of complications as severe as anaphylactic shock and transverse myelitis. Although these complications can now be virtually eliminated with preoperative antigen screening and diskography, the perceived risk has limited its continued use. More recent studies from experienced chymopapain users still tout chymopapain as a valuable adjunct to endoscopic disk surgery.15 The introduction of the operating microscope for diskectomy by Yasargil in 1967 and later by Williams encouraged smaller incisions for the standard posterior approach.16,17 The transcanal microscope-assisted technique became the gold standard; however, it still requires retraction of the dural tube and nerve, periosteal stripping of the muscle and ligaments, hemilaminotomy, and regional or general anesthesia. Tubular retractors have recently been developed that can be used with either a microscope or an endoscope for this posterior transcanal approach.18 This utilizes tissue dilation rather than cutting and minimizes the superficial tissue destruction, but it still requires the same amount of bone removal and neural manipulation as the standard microscopic posterior diskectomy. FIGURE 18–1 Kambin’s triangular working zone is the site of surgical access for posterolateral endoscopic disketomy. It is defined as a right triangle over the dorsolateral disk. The hypotenuse is the exiting nerve root, the base (width) is the superior border of the caudal vertebra, and the height is the dura/traversing nerve root. The concept of indirect decompression of the spinal canal via a posterolateral, extracanal approach was introduced by Kambin in 1973 using a Craig cannula for limited nucleotomy in combination with a transcanal approach.7,8 In 1975 Hijikata reported the first standalone nonvisualized posterolateral percutaneous central nucleotomy.6 Kambin went on to describe the safe triangular working zone (Kambin’s triangle) (Fig. 18–1) and results of arthroscopic microdiskectomy, in which arthroscopic visualization of the herniation via the posterolateral approach was used for diskectomy of contained herniations.5,7,8 Hermantin et al5 reported satisfactory results from video-assisted arthroscopic microdiskectomy in 97% of patients, compared with 93% in traditional microdiskectomy with an average of 31 months’ followup. The arthroscopic group had less narcotic use and less time off from work. The study was prospective and randomized, with 30 subjects in each group. Mayer and Brock also showed promising results in a prospective randomized study comparing percutaneous diskectomy with microscopic diskectomy for contained or slight subligamentous herniations.9 The percutaneous group showed comparable or superior results. Long-term disability, defined by return to work status, produced statistically significant differences. In the percutaneous group, 95% returned to their previous occupation, compared with 72.2% in the microdiskectomy group. Each group had 20 subjects. Evolving methodology in the 1980s and early 1990s allowed for endoscopic lumbar nerve root decompression by a visualized, direct excision of contained and noncontained herniated disk fragments.8,9,11,13 Yeung12 introduced a rigid rod-lens, flow-integrated, multichannel, wide-angle operating spinal endoscope in 1998 that allowed for even more flexibility accessing the disk and traversing and exiting nerve roots and the epidural space. The endoscope configuration offered significant visual improvement, and the complementary instrument system with specialized slotted and bevelended tubular access cannulas allowed for same-field viewing of the intradiskal space, annular wall, and epidural space. The Yeung Endoscopic Spine Surgery (YESS) technique allows for improved access to the posterior disk for visualized fragment resection, improved access to the undersurface of the superior articular facet for foraminoplasty, and protection of the neural structures by rotating the cannula.12 Indications The following are indications for employment of the YESS technique: Perhaps the ideal lesion for posterolateral selective endoscopic diskectomy is the far lateral extraforaminal disk herniation. The exiting nerve is routinely visualized, and the cannula inserts directly at the herniation site. This approach requires less manipulation of the exiting nerve root than the paramedian posterior approach. Any herniation contiguous with the disk space not sequestered and migrated is amenable to endoscopic disk excision. The timing of surgical treatment is similar to posterior transcanal diskectomy. The size and types of herniations chosen by the surgeon for endoscopic excision will depend on the skill and experience of the surgeon as well as the anatomic considerations in the patient relative to the location of the herniation. All contained disk herniations are certainly appropriate for endoscopic decompression. With experience, extruded herniations can be routinely addressed. The posterolateral endoscopic approach only requires tissue dilation to accommodate a 7 mm working cannula. This tissue-sparing approach offers consideration for earlier surgical timing when approach-related risk:benefit ratios are factored in after patients fail conservative treatment and continue to have debilitating pain without neurologic deficit. Quality of life issues and functional issues associated with chronic diskogenic pain can be addressed with this minimally invasive surgical option; therefore, small disk herniations with predominant leg pain, central disk herniations with predominant back pain, IDD, and annular tears causing chemical sciatica are amenable to disk surgery by endoscopic means. The diskectomy decompresses the disk, alleviating pressure on the anulus, and removes any unstable degenerated disk fragments that could herniate. Radiofrequency energy can be applied to the annular tears under direct visualization to contract the collagen and ablate ingrown granulation tissue, neoangiogenesis, and sensitized nociceptors. Interpositional nuclear tissue is frequently seen within the fibers of the annular tear, which prevents the tear from healing. This tissue can then be removed to allow the tear to heal. Endoscopic foraminoplasty can readily be achieved with bone trephines/rasps and the side-firing holmium: yttrium aluminium garnet (YAG) laser.19 The roof of the foramen is formed by the undersurface of the superior articular facet. This is easily visualized and accessed via the endoscope. The side-firing holmium: YAG laser and bone trephines strip the facet capsule and remove bone to enlarge the foraminal opening. Studies by Osman et al20 have demonstrated that decompression through the foramen can be more effective than posterior decompression for foraminal stenosis. The posterior removal of one third of the medial facet produces more instability than posterolateral foraminal decompression.20 Synovial cysts can also be visualized and removed. Diskitis can be treated with posterolateral endoscopic diskectomy and debridement. Current methods rely on needle aspiration followed by prolonged antibiotic treatment. Needle aspirations are not as reliable as tissue samples from endoscopic debridement and are often negative even in the face of bacterial diskitis. Surgeons are often hesitant to perform open debridement because of the morbidity of the open approach, creation of dead space and devascularized tissue, and concern for spreading the infection in the spinal canal. Endoscopic excisional biopsy and thorough debridement via the posterolateral portal have provided almost immediate pain relief and a much more reliable tissue sample for laboratory analysis and culture. Because only tissue dilation is used, no dead space is created that would allow the infection to spread. Many patients with diskitis have co-morbidities, which make them poor open surgical candidates. Surgical Equipment The Yeung Endoscopic Spine Surgery (YESS) system (Richard Wolf GmbH, Knittlingen, Germany) consists of the following instruments (Fig. 18–2): Adjunctive Equipment Adjunctive equipment for the YESS technique includes the following: Operating Room Setup Proper OR set up requires a radiolucent table with a hyperkyphotic frame, one C-arm, and a tower with the usual monitor for endoscopic viewing. The operating suite will ideally be equipped to record the procedure, including fluoroscopic images onto video and/or still images. Foot pedals controlling the radiofrequency probe, shaver, suction, C-arm, and laser should be ergonomically arranged. Required personnel include the anesthesiologist, scrub technician, circulator, C-arm technician, and a surgical assistant if a biportal approach is planned (Fig. 18–3). FIGURE 18–2 Partial instrument set for the Yeung Endoscopic Spine Surgery (YESS) technique. The patient is placed prone on the radiolucent hyperkyphotic frame (Kambin frame, US Surgical), with the arms away from the side of the body. Care is taken to line up the patient with the C-arm to ensure a perfect posteroanterior and lateral view on the fluoroscopy. The spinous processes should be centered between the pedicles on the posteroanterior (PA) view and the end plates parallel on the lateral view. The surgical level must be centered to avoid parallax error. Anesthesia consists of 0.5% local lidocaine infiltration, supplemented by Versed and fentanyl for conscious sedation. The sedation is kept light enough to allow feedback if the patient experiences any painful nerve root irritation. FIGURE 18–3 Proper operating room setup. Surgical Technique Protocol for Optimal Needle Placement Utilizing a thin metal rod as a radiopaque marker and ruler, lines are drawn on the skin to mark surface topography for guidance in free-hand biplane C-arm needle placement. These surface markings help identify three key landmarks for needle placement: the anatomic disk center, the annular foraminal window (centered within the medial and lateral borders of the pedicles), and the skin window (needle entry point) (Fig. 18–4). The skin window’s lateral location from the midline determines the trajectory angle into the foraminal annular window. Using the preceding method, a 45-degree trajectory to the disk should place the needle tip in the true anatomic disk center. This is good for a central nucleotomy to decompress the disk. Because most of the pathology being treated is located posteriorly, however, placement in the posterior one third of the disk is optimal. Thus, one needs to “fudge” 1 to 2 cm laterally for the optimal skin window placement to access the posterior one third of the disk. This allows one to avoid the facet joint with a shallower needle trajectory (~30 degrees in the coronal plane) to the disk. On the other hand, one can place the rod tip at the anterior portion of the disk when measuring the disk inclination plane on the lateral fluoroscopic view. This produces a longer measurement to the posterior skin plane, thus placing the skin window more lateral. This is the authors’ preferred method. This coordinate system of finding the optimal anatomical landmarks for instrument placement will help decrease the steep learning curve for needle placement and eliminate the less accurate “down the tunnel” method favored by radiologists and pain management physicians. The positive disk inclination plane of the L5–S1 disk is noteworthy. A steep positive inclination line (lordosis) will position the optimal skin window more cephalad from the transverse plane line, avoiding the high iliac crest. A flatly inclined L5–S1 disk will position the optimal skin window with the iliac crest obstructing the trajectory of the needle. The skin window will have to start more medial to avoid the iliac crest, and sometimes the lateral one fourth of the facet joint must be resected to allow for posterior needle placement in the disk. The first neutrally aligned disk inclination plane is usually at L4–L5 or L3–L4. A neutrally aligned disk inclination plane is in the same plane as the transverse plane line; thus, the skin window is in line with the transverse plane line. A negatively inclined disk, often at L1–L2 and L2–L3, places the skin window caudal to the transverse plane line. Needle Placement Infiltrate the skin window and subcutaneous tissue with 0.5% lidocaine. Insert a 6-inch-long, 18-gauge needle from the skin window at a 25- to 30-degree angle from the coronal plane (reciprocal of 60–65 degrees from the parasagittal plane), anteromedially toward the anatomical disk center. Infiltrate the needle tract with 0.5% lidocaine as you are advancing the needle. The superficial portion of the needle trajectory is usually outside the C-arm viewing perimeter. Once the needle tip is visible within the C-arm viewing perimeter, tilt the C-arm beam parallel to the disk inclination plane (the Ferguson view). This allows one to visualize the advancing needle in the true disk inclination plane. Advance the needle toward the target foraminal annular window. If minor directional adjustments are necessary, use the plane of the needle bevel and hub pressure to navigate. At the first bony resistance or before the needle tip is advanced medial to the pedicle, turn the C-arm to the lateral projection. Do not advance the needle tip medial to the pedicle during the initial approach. Doing so risks inadvertent traversing nerve root and dural puncture. FIGURE 18–4 Protocol for optimal needle placement. (A) Posteroanterior (PA) fluoroscopic view enables topographic location of the midline and the transverse disk plane. The intersection of these lines is the PA anatomic disk center. (B) Lateral fluoroscopic view enables topographic location of the disk inclination plane. (C) The inclination plane of each target disk is drawn on the skin from the lateral disk center. (D) The distance from the lateral disk center to the posterior skin plane is measured along the inclination plane. (E-F) This same distance is measured from the midline along the transverse disk plane for each target disk. At the end of this measure a line parallel to the midline is drawn to intersect the disk inclination line. This is the skin entry point, or “skin window,” for the needle. While monitoring the PA view, advance the needle tip through the anulus to the midline (anatomical disk center). Next, check the lateral view. If the needle tip is in the center of the disk on the lateral view, you have a central needle placement, which is good for a central nucleotomy. The needle tip ideally will be in the posterior one third of the disk, indicating posterior needle placement. This is ideal for accessing most herniations. Evocative Chromodiskography Perform confirmatory contrast diskography at this time. The following contrast mixture is used: 9.0 ml of Isovue 300 with 1.0 ml of indigo-carmine dye. This combination of contrast ratio gives readily visible radiopacity on the diskography image, and intraoperative light blue chromatization of pathologic nucleus and annular fissures, which help guide the targeted fragmentectomy. Chromodiskography is an integral part of PA selective endoscopic diskectomy. The indigo-carmine preferentially stains the acidic degenerated nucleus pulposus. This helps orient the surgeon to the endoscopic anatomy and selectively remove the herniated and unstable nucleus pulposus. The surgeon can follow the blue-stained tissue to the annular tears and the herniation tract. The ability of diskography to evoke a concordant painful response is also helpful to confirm the disk as a pain generator. The literature on diskography is currently considered controversial. It is controversial partly because of the high interobserver variability by diskographers in reporting the patient’s subjective pain as well as the ailing patient’s inability to give a clear response, especially if pain response is altered by the use of analgesics or sedation during the procedure. The surgeon who is accomplished in endoscopic spine surgery should do the diskography to decrease the interobserver variability in interpreting the patient’s response and thus better select for appropriate patients. Instrument Placement Insert a long, thin guidewire through the 18-gauge needle channel and into the disk. Remove the needle, and slide the bluntly tapered tissue dilating obturator over the guidewire until the tip of the obturator is firmly engaged in the annular window. An eccentric parallel channel in the obturator allows for four-quadrant annular infiltration, using small incremental volumes of 0.5% lidocaine in each quadrant, which is enough to anesthetize the anulus, but not the spinal nerves. Hold the obturator firmly against the annular window surface, and remove the guidewire. Infiltrate the full thickness of the anulus through the obturator’s center channel using lidocaine. The next step is the through-and-through fenestration of the annular window by advancing the bluntly tapered obturator with a mallet. Annular fenestration is the most painful step of the entire procedure. Advise the anesthesiologist to heighten the sedation level just prior to annular fenestration. Advance the obturator tip deep into the anulus, and confirm on the C-arm views. Now slide the beveled access cannula over the obturator toward the disk. Advance the cannula until the beveled tip is deep in the annular window. Remove the obturator, and insert the endoscope to get a view of the disk nucleus and anulus. On the other hand, if you are worried about further extruding a large disk herniation, or if you want to inspect the outer annular fibers before fenestrating the anulus, you can engage the outer anulus with the blunt obturator. Next, advance the beveled cannula over the obturator to the anulus. Remove the obturator, and insert the endoscope. The outer annular fibers can be inspected to ensure that no neural structures are in the path of the cannula prior to the annulotomy. An annulotome or a cutting trephine can then be used for the annular fenestration under direct vision. Prominent disk tissue can be removed prior to entering the disk with the cannula. The foraminal annular window is an easily identifiable C-arm and intraoperative anatomical landmark, and is the starting location for endoscopic disk excision. Through the endoscope, you may see various amounts of blue-stained nucleus pulposus. The general-purpose access cannula has a bevel hypotenuse of 12 mm and outside diameter of 7 mm. When the cannula is slightly retracted to the midstraddle position in relationship to the annular wall, the wide-angle scope visualizes the epidural space, annular wall, and intradiskal space in the same field. Performing the Diskectomy The basic endoscopic method to excise a noncontained paramedian extruded lumbar herniated disk via a uniportal technique is described here. First, enlarge the annulotomy medially to the base of the herniation with a cutting forcep. The side-firing holmium:YAG laser can also be used to enlarge and widen the annulotomy. This is performed to release the annular fibers at the herniation site that may pinch off or prevent the extruded portion of the herniation from being extracted. A large amount of blue-stained nucleus is usually present directly under the herniation apex, which can be likened to the submerged portion of an iceberg. The nucleus here represents migrated and unstable nucleus. The endoscopic rongeurs are used to extract the bluestained nucleus pulposus under direct visualization (Fig. 18–5). The larger straight and hinged rongeurs are used directly through the cannula after the endoscope is removed. Fluoroscopy and surgeon feel guide this step. By grabbing the base of the herniated fragment, one can usually extract the extruded portion of the herniation. Initial medialization and widening of the annulotomy reduce the prospect of breaking off the apex of the herniation. The traversing nerve root is readily visualized after removal of the extruded herniation (Figs. 18–6, 18–7, 18–8). FIGURE 18–5 Uniportal technique for selective endoscopic diskectomy. Rongeurs are used for visualized fragmentectomy. The beveled cannula can be positioned to view the intradiskal cavity, annular wall, and epidural space in the same field of vision. FIGURE 18–6 Endoscopic visualization of a right-sided foraminal L4–L5 herniated nucleus pulposus causing pressure on the inflamed exiting nerve root. The herniated nucleus is stained blue with indigo-carmine, which allows for improved targeted fragmentectomy. The top of the picture is dorsal, and the right is cephalad. FIGURE 18–7 Endoscopic view of the removal of bluestained herniated nucleus pulposus just underneath the traversing nerve root. Visualization of the traversing nerve root is blocked by the rongeur and disk fragment in this view. The attenuated unstained annular fibers can be seen dorsally and surrounding the blue-stained nucleus pulposus. Next, perform bulk decompression by using a straight and flexible suction-irrigation shaver (Endius MDS). This step requires C-arm localization of the shaver head before power is activated to avoid nerve/dura injury and anterior annular penetration. The cavity thus created is called the working cavity. The debulking process serves two functions. First, it decompresses the disk, reducing the risk for further acute herniation. Second, it removes the unstable nucleus material to prevent future reherniation. Inspect the working cavity. If a noncontained extruded disk fragment is still present, as indicated by the presence of blue-stained nucleus material posteriorly, then these fragments are teased into the working cavity with the endoscopic rongeurs and the flexible radiofrequency trigger-flex bipolar probe (Ellman) and removed. Creation of the working cavity allows the herniated disk tissue to follow the path of least resistance into the cavity. The flexible radiofrequency bipolar probe is used to contract and thicken the annular collagen at the herniation site. It is also used for hemostasis throughout the case. FIGURE 18–8 The same endoscopic view as Figure 18–7 after complete removal of the herniation. The traversing nerve root is clearly visualized and is no longer compressed. The vast majority of herniations can be treated via the uniportal technique. For large central herniations, the disk sometimes needs to be approached from both sides (i.e., a biportal technique). Complications and Avoidance As with arthroscopic knee surgery, the risk of serious complications or injury is low, ~1 to 3% in our experience. As with any surgery, the usual risks of infection, nerve injury, dural tears, bleeding, and scar formation are always present. Dysesthesia, the most common postoperative complaint, occurs ~5 to 15% of the time, and is almost always transient. Its cause is still incompletely understood and may be related to nerve recovery, operating adjacent to the dorsal root ganglion of the exiting nerve, or a small hematoma adjacent to the ganglion of the exiting nerve because it can occur days or even weeks after surgery. Transient dysesthesia can occur even in cases where no adverse events were detected with continuous electromyography (EMG) and SEP neuromonitoring; therefore, it cannot be completely avoided. The symptoms are like a variant of complex regional pain syndrome (CRPS), but less severe, and without the skin changes that accompany CRPS. Dysesthesia is readily treated by transforaminal epidural blocks, rarely sympathetic blocks, and the use of Neurontin titrated up to 1800 to 3200 mg/day if needed. Bowel injury or large vessel injury is extremely rare, but it is possible if the suction-irrigation shaver penetrates the contralateral anulus from an incompetent anulus or from a defect left after removing a disk herniation that has extruded through the annular wall. Careful intraoperative fluoroscopic localization and “ feeling” the contralateral anulus with the instrument prior to activating it will help prevent the shaver from advancing past the contralateral annular wall. Avoidance of complications is enhanced by the ability to visualize clearly normal and pathoanatomy, the use of local anesthesia and conscious sedation rather than general or spinal anesthesia, and the use of a standardized needle placement protocol. The entire procedure is usually accomplished with the patient remaining comfortable during the entire procedure and should be done without the patient feeling severe pain, except when expected (e.g., during evocative diskography, annular fenestration, or when instruments are manipulated past the exiting nerve). Local anesthesia using 0.5% lidocaine allows generous use of this dilute anesthetic for pain control and still allows the patient to feel pain when the nerve root is manipulated. Continuous EMG and SEP can also help monitor and prevent nerve irritation. This usually correlates well with patients’ intraoperative feedback. Discussion Endoscopic spine surgery has a very high learning curve, but it is within the grasp of every endoscopic surgeon with proper training. As with any new procedure, the complication rate may be higher during the learning curve and may vary with each surgeon’s skills and experience. The endoscopic technique is safer for the patient because he or she is conscious and able to provide immediate input to the surgeon when pain is generated. The surgeon’s ability to perform the surgery without causing the patient undue pain will self-select for surgeons who can master the technique to the extent that the surgeon will prefer endoscopic over traditional surgery for the same condition. For most contained disk herniations and diskogenic pain, the experienced endoscopic spine surgeon will opt for the endoscopic approach as the treatment of choice. Case Illustration A 22-year-old male with a 2-year history of low back pain and intermittent right leg pain sustained an acute worsening of his right leg pain 12 days prior to evaluation. He proportionalized his pain to 5% back and 95% leg pain. He complained of a new onset of weakness, tingling, and constant numbness. The pain and numbness radiated down the posterolateral leg to the dorsum of the right foot. He was unable to bear weight on the right leg and was using a walking pole for support. He was unable to sleep supine and had to sleep in a recliner to minimize the pain. Sitting provided some relief. He denied bowel or bladder incontinence, but had constipation for the last 12 days. Physical exam revealed a limited lumbar extension to 10 degrees, tenderness in the right sciatic notch, positive straight leg raising (SLR) and LasegueȲs tests, positive contralateral SLR, 2+ bilateral patella and Achilles deep tendon reflexes, decreased sensation to light touch over the dorsum of the foot and to a lesser extent the lateral border of the foot, and weakness. The right-sided weakness was graded as anterior tibialis, external hallucis longus (EHL), hip abductor, gastrocnemius soleus. FIGURE 18–9 Preoperative axial (A) and sagittal (B) MRI revealed a large right paracentral/foraminal HNP causing compression on the exiting and traversing nerve roots. Other axial cuts showed migration caudally, but the fragment appeared confluent with the base of the herniation. FIGURE 18–10 Postoperative MRI—(A) atrial and (B) sagittal—revealed excellent removal of the herniated disk and decompression of the nerve roots. The instrument trajectory can be seen within the disk as an area of higher signal on the T2-weighted image. MRI revealed a large right paracentral/foraminal extruded herniated nucleus pulposus with slight caudal migration, causing compression of both the exiting and traversing nerve roots (Fig. 18–9 ). Surgery was recommended due to the acute onset and progressive neurologic deficits. After a full discussion of his risks, benefits, and alternatives, the patient elected to undergo outpatient selective endoscopic posterolateral diskectomy. The patient experienced better than 80% pain relief immediately postop. He had some mild dysesthetic burning over the L4 distribution that started a few days postop. This completely resolved by 4 weeks with the aid of Neurontin 300 mg TID. A postoperative MRI was ordered when the patient had acute worsening of his leg pain 11 days postop (Fig. 18–10). He said he “overdid it.” The patient’s leg weakness was improving, but because some weakness was still present, we wanted to make sure he did not have a recurrent herniation. The MRI revealed excellent herniation removal without any retained fragments. The patient’s acute pain resolved within 24 hours, and he had no pain at all by 4 weeks. His weakness continued to improve grading EHL, 5/5 hip abductor, 4+/5 anterior tibialis, and 5/5 gastrocnemius soleus at his last follow-up 8 months postoperatively. Posterolateral endoscopic diskectomy provides excellent access to the epidural space from pedicle to pedicle, facilitating removal of extruded or migrated fragments, in addition to contained disks. The YESS technique is performed with tissue dilatation to accommodate a 7 mm working cannula. It avoids the significant trauma of muscle stripping, muscle retraction, bony resection, and nerve root retraction associated with standard open surgery. Because the patient is conscious throughout the procedure, it is safer than the open method by preventing nerve root injury. REFERENCES 3. Adams MA, Hutton WC. Gradual disc prolapse. Spine. 1985;10: 524–531. 4. Adams MA, Hutton WC. Prolapsed intervertebral disc-hyperflexion injury. Spine. 1982;7:184–191. 14. Gogan WJ, Fraser RD. Chymopapain: a 10-year, double blind study. Spine. 1992;17:388–394.
18

Posterolateral Selective Endoscopic Diskectomy: The YESS Technique

< div class='tao-gold-member'>
Posterolateral Selective Endoscopic Diskectomy: The YESS Technique
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








