Fig. 2.1
Patients (n = 197) for deep-brain stimulation. STN Subthalamic nucleus, VIM ventral intermediate nucleus, GPI globus pallidus internus
Table 2.1
Parameter settings for deep-brain stimulation
Parameter | VIM | GPI | STN |
|---|---|---|---|
Frequency | 144 | 156 | 133 |
Pulse width (μs) | 196 | 203 | 98 |
Amplitude (V) | 2.9 | 3.1 | 3.1 |
Output (μA) | 83 | 140 | 58 |
Impedance (Ω) | 1,290 | 740 | 997 |
Statistical evaluation of the results was done by multivariate analysis using the one-way ANOVA rank sum test (Sigmastat 1.0, Jandel Scientific, Chicago, IL, USA).
2.3 Motor Outcome and Complications
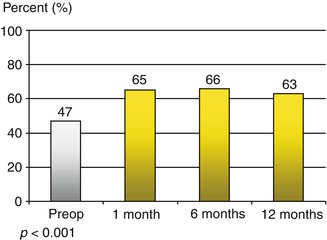
Suppression tremor was similarly successful for resting and postural tremor as well as for action-intention tremor. The etiology the tremor did not affect the effectiveness DBS, although other symptoms persisted in multiple sclerosis patients (Fig. 2.2). The motor symptoms in Parkinson patients also improved significantly (Fig. 2.3). The Parkinson patients were assessed both “on” and “off” medication as well as “on” and “off” stimulation. Surgery-related adverse events in the form of transient disorientation during bilateral STN stimulation occurred in 16 patients (13 %). In two of them (1.8 %), the symptoms persisted for some time and required treatment with antipsychotic drugs. Infections were seen in five patients (2.5 %). In these cases, the stimulation systems had to be removed and were reimplanted after 6 months. The positive effect of stimulation could be restored in all patients. Intracerebral hemorrhages occurred in two patients. One of them developed permanent contralateral hemiplegia; the other resulted in a permanent neuropsychological deficit (Fig. 2.4).
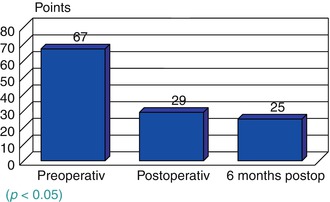
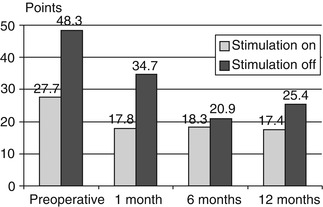

Fig. 2.2
Tremor scale according to Fahn [9] in all patients, significant differences between pre- and postoperative state (p < 0.001, Wilcoxon’s signed-rank test)

Fig. 2.3
Unified Parkinson’s Disease Rating Scale (UPDRS) scores (medication on; p < 0.001)
2.4 Discussion
DBS has been shown to be effective in treating the motor symptoms in patients with extrapyramidal movement disorders. Tremor of different origin was suppressed by stimulation of the VIM and STN alike. VIM stimulation and assessment using the ETRS were found to be suitable for essential tremor and cerebellar tremor [66]. Stimulation of the STN should be aimed at in Parkinson patients with tremor as the predominant manifestation because of potential progression of the other motor symptoms, on which VIM stimulation would have no effect. The Parkinson patients were assessed by means of the UPDRS. They showed significant and permanent improvement in part III of the scale (motor score). Their quality of life also improved considerably on a long-term basis.
Chronic DBS has led to a renaissance of functional neurosurgery. Numerous procedures have been performed in recent years to treat patients with extrapyramidal movement disorders. New methods have decisively improved the targeting accuracy and success rates of stereotactic interventions and led to new indications for such procedures [61].
The effectiveness of DBS has been confirmed in numerous controlled studies. Adverse events or severe complications are rare. With the advances made in neurophysiologic techniques and imaging modalities, the targets in the basal ganglia can be determined even more reliably. The patients’ quality of life is improved effectively and permanently. A German multicenter study is currently investigating a potential neuroprotective effect of DBS and its role in the early period of Parkinson’s disease.
The indication for DBS may be extended to other neurological disorders such as epilepsy, as suggested by some promising case reports. Studies aimed at investigating this indication in larger patient groups are being prepared.
2.5 Summary and Conclusion
The developments in DBS techniques have led to a significant advancement in the treatment of extrapyramidal motor symptoms. Both tremor and rigidity as well as akinesia can be permanently suppressed by introducing a high-frequency current into different basal ganglia nuclei in diseases like Parkinson’s and essential tremor.
Continuous DBS is performed by means of stereotactically implanted quadripolar electrodes in the ventrolateral thalamus, the GPI, or the STN. The current for electrostimulation is supplied by a pulse generator that is implanted subcutaneously in the infraclavicular region in a second procedure (Soletra/Itrel II for unilateral stimulation, Kinetra for bilateral stimulation, Medtronic, USA). The generator is implanted after correct positioning of the electrodes, and their effectiveness has been confirmed by external stimulation. In order to demonstrate the potentials and limitation of DBS in Parkinson’s disease, the results of 110 patients of our center that were treated with DBS and followed up using standardized rating scales before and after surgery are provided (UPDRS, BFMDRS, and ETRS) [9, 17].
Extrapyramidal symptoms like tremor, rigidity, akinesia, and dystonia are efficiently suppressed by DBS. Further studies are ongoing to explore the potential of DBS in treating other types of movement disorders.
Functional neurosurgery has regained significance with the advent of new treatment strategies for various neurological disorders. Continuous high-frequency stimulation effectively and permanently suppresses symptoms of extrapyramidal movement disorders such as tremor, rigidity, akinesia, and dystonia.
References
1.
2.
3.
Aziz TZ, Bain PG (1999) Deep brain stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 67:281PubMedCentralPubMedCrossRef
4.
5.
Benabid AL (1999) History of stereotaxis. Rev Neurol (Paris) 155:869–877
6.
Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Pollak P (1998) Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord 13(Suppl 3):119–125PubMed
7.
9.
10.
11.
Caparros-Lefebvre D, Blond S, N’guyen JP, Pollak P, Benabid AL (1999) Chronic deep brain stimulation for movement disorders. Adv Tech Stand Neurosurg 25:61–136PubMed
13.
Davis KD, Tasker RR, Kiss ZH, Hutchison WD, Dostrovsky JO (1995) Visceral pain evoked by thalamic microstimulation in humans. Neuroreport 6:369–374PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree