Chapter 47 Principles of Endovascular Therapy
Ischemic Stroke
Acute Stroke Treatment
Stroke is the third leading cause of death and the most common reason for disability worldwide (Rosamond et al., 2008). The majority of ischemic strokes result from vessel occlusion secondary to thromboembolic or atheromatous processes (Sussman and Fitch, 1958). To date, timely revascularization and reperfusion of ischemic brain tissue is the most effective therapeutic strategy. Intravenous (IV) thrombolysis has been restricted to a very narrow 3-hour time window based on the NINDS trials (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). Using modified clinical selection criteria, the third European Cooperative Acute Stroke Study recently demonstrated a benefit of IV thrombolysis up to 4.5 hours after symptom onset (Hacke et al., 2008). Nonetheless, the main restrictions on IV recombinant tissue plasminogen activator (rtPA) remain the narrow time window for administration and the nondiscerning systemic mode of delivery. Coupled with an insufficient public awareness, these factors have limited the use of IV thrombolysis to less than 5% of eligible candidates (Barber et al., 2001). Endovascular methods of acute stroke treatment have been developed to help address these shortcomings.
Although inclusion criteria vary for each of the endovascular acute stroke trials, careful patient selection and prudent utilization of recanalization therapies are critical. Treatment algorithms are predicated on clinical and radiographic criteria. Precise assessment of the National Institutes of Health Stroke Scale (NIHSS) (Box 47.1) and time of symptom onset determines risk/benefit stratification. Axial imaging (computed tomography [CT], magnetic resonance imaging [MRI]) is used to quantify infarct size and screen for hemorrhage. CT or MR angiography (CTA, MRA) can determine vessel patency, whereas perfusion-weighted MRI helps characterize salvageable tissue in the ischemic penumbra.
Box 47.1 National Institutes of Health Stroke Scale
Intraarterial Thrombolysis
Compared to IV approaches, intraarterial (IA) administration of thrombolytics via a microcatheter positioned in the cerebral vasculature affords rapid local delivery of greater therapeutic treatment concentrations to the site of vascular occlusion. The selective nature of the delivery system maximizes lysis while limiting systemic conversion and resultant hemorrhage. Intraarterial thrombolysis represents a targeted strategy that lengthens the total therapeutic window for acute stroke intervention. The technique entails performing a catheter-based cerebral angiogram to confirm the point of occlusion. Under fluoroscopic guidance, a microcatheter is advanced though a larger guide catheter to the base of, into, or distal to the clot. Once positioned, a thrombolytic agent is injected over a 1- to 2-hour time period as intermittent control angiograms are performed (Gandhi et al., 2009). Clinical trials have been performed using streptokinase, urokinase, rtPA, recombinant prourokinase, and reteplase. These agents differ in fibrin selectivity, stability, half-life, and mechanism of action (Gandhi et al., 2009; Schellinger et al., 2001). The Prolyse in Acute Cerebral Thromboembolism II (PROACT II) trial demonstrated the effectiveness of IA prourokinase when given within 6 hours of acute stroke caused by middle cerebral artery occlusion (Furlan et al., 1999). Patients were randomized to IA recombinant prourokinase and IV heparin or heparin alone. The primary clinical outcome, the proportion of patients with minimal or no disability at 90 days (modified Rankin Scale [mRS] score ≤ 2), was achieved in 40% of the treatment group (patients administered heparin and IA prourokinase) and 25% of the controls (patients administered heparin only).
Although IA thrombolysis is an effective treatment, the time delay required for cerebral angiography and microcatheter positioning is an inherent shortcoming. To address this, bridging strategies have employed a combined IV/IA approach. The first such trial was the Emergency Management of Stroke Bridging Trial (Lewandowski et al., 1999). This was a double-blind randomized placebo-controlled phase-I trial of IV rtPA or placebo followed by immediate IA treatment with rtPA. Although recanalization was significantly greater in the IV/IA rtPA group, there was no difference in 3-month functional outcomes, and an increased incidence of death was observed in that treatment group.
The Interventional Management of Stroke (IMS) study investigators compared acute stroke patients treated with a bridging IV rtPA dose (0.6 mg/kg) followed by IA rtPA, with historical controls given only the traditional IV dosing regimen from the NINDS trial. The IMS I study was an open-label single-arm feasibility study using a standard microcatheter to infuse a total dose of up to 22 mg of IA rtPA over 2 hours (IMS Study Investigators, 2004). The objective of the second IMS study (IMS II) was to continue investigating the feasibility of a combined IV/IA approach, permitting the use of the EKOS MicroLysUS (EKOS Corp., Bothell, Washington) microinfusion catheter, which incorporates sonographic technology to increase permeability and penetration of the intraluminal clot (IMS II Trial Investigators, 2007). The IMS study cohorts demonstrated better functional outcomes and a decreased mortality rate compared to historic controls. The rate of symptomatic intracranial hemorrhage was similar to that of the IV rtPA group in the NINDS trial (IMS Study Investigators, 2004; IMS II Trial Investigators, 2007; National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). These data suggest that bridging protocols combining IV therapy and subsequent endovascular procedures may improve recanalization rates and functional outcome following acute stroke (Mazighi et al., 2009). Definitive evidence to support this combination may not be available, however, until the prospective randomized IMS III trial is completed.
Mechanical Recanalization
Although outcomes following acute stroke have improved with the administration of IV/IA thrombolytics, there are many patients who are not candidates for these treatment schemes (Furlan et al., 1999; Williams et al., 2009). Narrow time windows and the risk of hemorrhage associated with thrombolytic agents have prompted the design and application of mechanical thrombectomy devices. Endovascular clot retrieval provides potential for rapid restoration of flow, with a decreased incidence of clot fragmentation and distal embolism (Nogueira et al., 2009). Catheter-based retrieval/aspiration systems are used in acute stroke patients who are either ineligible for or have failed IV rtPA administration; they may be used in conjunction with thrombolytic infusion. Clot-retrieval devices have effectively lengthened the treatment window for acute stroke.
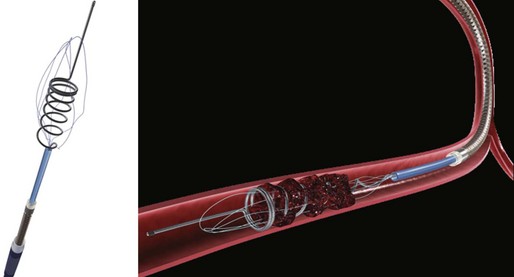
The first device to have received U.S. Food and Drug Administration (FDA) approval for IA stroke treatment is the MERCI clot retriever (Concentric Medical Inc., Mountain View, California). This retrieval system includes the MERCI retrieval device, a compatible microcatheter, and a balloon guide catheter. The retriever is a flexible tapered nitinol wire with radiopaque helical loops at the distal tip (Fig. 47.1). Newer-generation retrievers (L series and V series) have cylindrical rather than tapered loops (X series) and a bound suture material that enhances clot capture. An 8F or 9F balloon guide catheter is advanced into a proximal vessel and inflated to cease anterograde blood flow and thus prevent distal emboli during clot extraction. The microcatheter is advanced coaxially over a microguidewire into the target vessel and through the clot. Once situated in the distal vessel, the microguidewire is exchanged for the clot retriever. The device is unsheathed to position several loops distal to the clot, and the retriever/microcatheter unit is then withdrawn into the balloon guide catheter under constant manual suction (Fig. 47.2).
Results from the nonrandomized Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial demonstrated safety and efficacy of the X-series clot retrieval system in restoring the patency of occluded intracranial vessels within the first 8 hours of acute stroke (Smith et al., 2005). Some 48% of occluded vessels were recanalized, a rate significantly higher than that of the control arm in the PROACT II trial (18%) (Furlan et al., 1999; Nogueira et al., 2009b). After adjuvant therapy (IA rtPA, angioplasty, snare), the rate of recanalization was 60.3%. The overall rates of good outcome (mRS score ≤2) and mortality were 27.7% and 43.5%, respectively, with a clinically significant procedural complication rate of 7.1%. Revascularization was found to be an independent predictor of decreased mortality and favorable neurological outcome at 90 days.
More recently, the Multi-MERCI Stroke Trial was conducted. This was an international multicenter, prospective, single-arm trial similar to the previous MERCI trial (Smith et al., 2008). However, this study included patients receiving IV rtPA with persistent arterial occlusion and allowed utilization of a newer-generation retrieval device (L5). Treatment with the retriever alone resulted in successful recanalization of 55% of treatable vessels. Recanalization rate was 68% after adjuvant therapy. The overall rates of good outcome (36%) and mortality (34%) were substantially improved when compared to those of the original MERCI trial. Once again, good outcomes were more frequent and mortality was lower with successful recanalization. The authors concluded that mechanical thrombectomy after IV rtPA seems as safe as mechanical thrombectomy alone, and that thrombectomy is efficacious in opening intracranial vessels during acute stroke in patients who are either ineligible for or have failed to recanalize with IV rtPA.
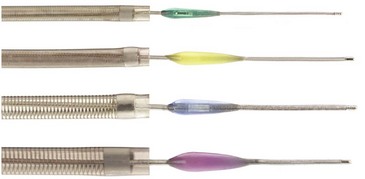
The Penumbra System (Penumbra Inc., Alameda, California) is an aspiration device through which a thromboembolic clot can be retrieved following acute stroke (Fig. 47.3). The device removes thrombus via aspiration, mechanical disruption, and extraction. Multiple aspiration catheters of varying luminal diameters are available for use in the cervical and intracranial vasculature, depending on vessel caliber. The aspiration device is advanced coaxially to the level of the thrombus through a guide catheter. Once positioned just proximal to the target lesion, an aspiration pump is connected to the reperfusion catheter. The separator is then advanced through the catheter into the distal clot and repeatedly retracted and advanced, aiding in the debulking process. A multicenter prospective, single-arm, phase I trial of the Penumbra reperfusion catheter was designed to assess safety and efficacy (Bose et al., 2008). The primary endpoint was revascularization, defined by a Thrombolysis in Myocardial Infarction (TIMI) score of 2 or 3. Recanalization was achieved in all vessels in which the device was deployed. A secondary endpoint of mRS score of 2 or less or improvement of NIHSS score by 4 or better was achieved in 45% of patients. The Penumbra device has recently received FDA approval (Fig. 47.4).
No other mechanical embolectomy device is currently approved by the FDA for use in acute stroke. The Phenox Clot Retriever (Phenox GmbH, Bochum, Germany), Neuronet Endovascular Snare (Guidant Corp., Temecula, California), Catch Mechanical Thrombus Retriever (Balt, Montmorency, France), Alligator retriever (Chestnut Medical Technologies Inc., Menlo Park, California), Amplatz GooseNeck Snare (ev3 Inc., Irvine, California), and TriSpan device (Boston Scientific, Natick, Massachusetts) have all been used successfully in a limited number of acute stroke patients, without large-scale demonstration of safety or efficacy (Gandhi et al., 2009; Katz et al., 2006; Nogueira et al., 2009a).
Angioplasty and Stenting
Despite increased experience with IA thrombolytic administration and technical advancements in the development of embolic retrieval or aspiration devices, many intracranial thrombi remain recalcitrant to thrombolysis or embolectomy. Mature fibrinous clots are often difficult to treat pharmacologically, and thrombi adherent to the vascular intima or associated with underlying atheromatous disease pose inherent structural risks to mechanical embolectomy procedures. Percutaneous transluminal angioplasty (PTA) and stenting procedures have been successful in acute coronary revascularization. Recanalization in the absence of clot dissolution or extraction may be sufficient for successful acute stroke treatment (Levy et al., 2009). Several small studies have reported improvement in clinical outcome following angioplasty and thrombolytic administration in the setting of acute stroke (Nogueira et al., 2008; Ringer et al., 2001; Yoneyama et al., 2002). Recently a single-arm prospective trial of primary intracranial stenting for acute stroke demonstrated promising results. The authors reported 100% TIMI 2-3 recanalization in a cohort of 20 patients treated with intracranial stenting. Self-expanding stents (Wingspan [Boston Scientific, Natick, Massachusetts]; Enterprise [Cordis Neurovascular Inc., Miami Lakes, Florida]) designed exclusively for cerebral indications facilitated access and navigation through tortuous craniocervical anatomy. Although adjuvant IA antiplatelet therapy was used in 50% of patients, the authors reported only a 5% rate of symptomatic intracranial hemorrhage. At 1 month, 60% of patients had mRS scores of 3 or below, and 45% had mRS scores of 1 or below. These results are encouraging. Larger studies are needed, however, to adequately assess the safety and efficacy of this treatment modality.
Alternative Reperfusion Strategies
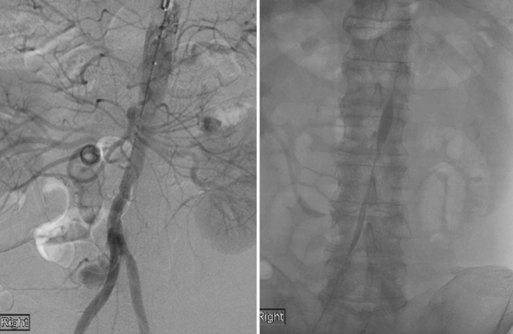
Alternatives to recanalization strategies focus on collateral perfusion as well as flow reversal following acute stroke. One such mechanical approach to flow augmentation is the NeuroFlo catheter (CoAxia Inc., Maple Grove, Minnesota), a dual-balloon catheter designed for partial occlusion of the descending aorta, above and below the renal arteries (Fig. 47.5). Temporary partial aortic occlusion is thought to augment cerebral blood flow through mechanical flow diversion. Multicenter clinical trials are currently being conducted to assess the safety and efficacy of this treatment modality (Liebeskind, 2010; Nogueira et al., 2009a; Uflacker et al., 2008). If efficacious, such alternate reperfusion strategies may serve to supplement existing recanalization treatments or represent a novel paradigm for patients ineligible for direct revascularization.
Carotid Artery Disease
The two large landmark randomized controlled trials that enrolled symptomatic carotid stenosis patients were the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST) (European Carotid Surgery Trialists’ Collaborative Group, 1996; Barnett et al., 1998). The NASCET investigators revealed that carotid endarterectomy reduced the risk of ipsilateral stroke (9%) at 2 years, compared to best medical therapy (26%) in patients harboring carotid stenoses of 70% to 99%. A more modest yet still significant reduction in the 5-year rate of ipsilateral stroke was evident in patients with 50% to 70% stenoses. The overall perioperative stroke and death rate for patients treated with CEA was 5.8%. Using a technically varied measurement criterion for quantifying luminal stenosis, the ECST arrived at a similar result. Data demonstrated that surgically treated patients with stenoses greater than 80% had a lower estimated risk of death or major stroke when compared to those managed medically.
The Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST) addressed indications for CEA in asymptomatic patients (Executive Committee for the Asymptomatic Carotid Atherosclerosis Study, 1995; Halliday et al., 2004). Both studies demonstrated that compared to those treated with best medical therapy, the 5-year incidence of ipsilateral stroke or death was reduced in the carotid endarterectomy group. The degree of benefit for asymptomatic patients was substantially less than previously documented in symptomatic individuals. The 5-year stroke and death rate was 11% for patients in the medical therapy cohort, while the stroke risk was 5.1% for patients treated with CEA. The benefit was evident predominantly in men.
Based on such results, the American Heart Association guidelines recommend CEA for symptomatic patients with 50% to 99% stenosis if the perioperative risk of stroke or death is less than 6%, and for asymptomatic patients with stenoses of 60% to 99% if the perioperative risk of stroke or death is less than 3% (Biller et al., 1998; Sacco et al., 2006).
Carotid Artery Angioplasty and Stenting
Angioplasty and Stenting Procedure
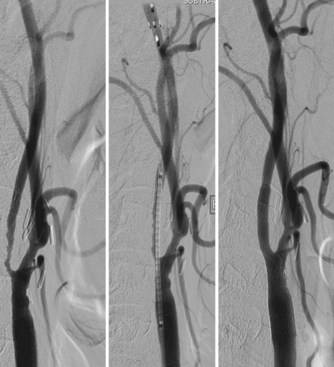
Next, embolic protection is secured in all attainable cases. Carotid plaques may be extremely friable, and even minor manipulation can result in distal embolization or vessel occlusion. Cerebral protection devices of varying construction are used to prevent embolic complications during CAS procedures. Most commonly, over-the-wire distal filters measured to the diameter of the native cervical internal carotid artery (ICA) are navigated past the stenotic lesion to capture embolic material (Fig. 47.6). Such devices allow continuous cerebral perfusion through pores in the filter baskets. Following angioplasty and stenting, the device is collapsed and withdrawn and embolic material retrieved. Alternatively, balloon occlusion devices may be used to completely prevent anterograde flow within the ICA. Any displaced material can be aspirated prior to balloon deflation. However, temporary carotid occlusion may not be well tolerated in all patients, limiting the applicability and utility of this strategy. Guide catheters with distal occlusion balloons may be inflated in the common carotid artery to achieve proximal control and distal flow reversal. Concomitant balloon inflation in the external carotid artery results in more thorough protection. Temporary proximal occlusion is generally performed when distal deployment of an embolic protective device (EPD) is not feasible owing to vessel size, tortuosity, or other anatomical restrictions.
Once embolic protection is achieved, balloon predilatation of tight stenoses can help ensure safe advancement of a self-expanding stent past the target lesion. Balloon inflation is done slowly to lessen the incidence of thromboembolism. Deflation must also be undertaken in a controlled, deliberate fashion. An experienced anesthesiologist and open communication are critical during this portion of the procedure. The endovascular surgeon must inform the anesthesia team of possible bradycardia associated with balloon inflation. This way, changes in the heart rate can be managed in a timely and proper fashion during angioplasty. Stent diameter is calibrated to 100% of the common carotid artery diameter, and length is measured to traverse the entire lesion with a margin of several millimeters both proximally and distally. Tapered stents are available to accommodate the larger diameter of the common carotid artery and the smaller lumen of the ICA. Depending on residual stenosis and distal perfusion, post-stent balloon dilatation may be undertaken. It is critical that balloon angioplasty remain within the confines of the stented arterial segment to prevent dissection of unprotected vascular intima (Fig. 47.7).
Clinical Trials
When carotid angioplasty procedures were initially introduced more than 25 years ago, they were performed without stenting or distal embolic protection. Despite these limitations, early data suggested the promise of percutaneous intervention (Yadav et al., 1997). The first multicenter randomized trial that compared carotid angioplasty and CEA was the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS Investigators, 2001). This trial enrolled symptomatic patients with clinically significant (>80%) ICA stenosis. Only 26% of patients (those enrolled later in the study) underwent stenting in addition to transluminal angioplasty. Nonetheless, the 30-day risk of death or stroke was similar between the percutaneous and surgical groups.
The first multicenter randomized CAS study in the United States, the Carotid Wallstent Trial, was conducted using the tracheobronchial Wallstent device (Boston Scientific, Natick, Massachusetts) in symptomatic patients deemed standard risk for carotid endarterectomy. The trial was terminated prematurely, and although the outcomes for stenting and surgery were not statistically different, a strong trend favored surgery (Alberts, 2001). Many believe that relatively limited operator experience and early-generation nondedicated devices profoundly affected the outcome of the stenting cohort in this investigation. The efficacy of a nascent technique was being compared to that of a refined surgical procedure.
Through the 1990s, dedicated improved stent implants and EPDs were developed. Randomized CAS trials and registries targeted patients at high risk for endarterectomy, a cohort of individuals for which previous data were either lacking or revealed suboptimal surgical outcomes, The multicenter randomized Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial enrolled high-risk symptomatic and asymptomatic patients (Yadav, 2004). The primary endpoint (death, stroke, and myocardial infarction (MI) at 30 days, plus ipsilateral stroke or neurological death at 1 year) occurred at a rate of 12.2% for stenting and 20.1% for surgery patients (P = 0.05). In addition to achieving noninferiority of the primary outcome, the study demonstrated significant decrease in target-vessel revascularization (repeat procedures) and cranial nerve palsies in the CAS cohort (P <0.05). On the basis of these data, the FDA recommended approval of the Cordis PRECISE stent and ANGIOGUARD distal filter device (Cordis Corp., Miami Lakes, Florida) for symptomatic patients at high risk for CEA. Three-year follow-up data demonstrated no significant difference in long-term outcomes between CAS and CEA (Gurm et al., 2008). Following the SAPPHIRE trial, a series of multicenter registries was undertaken to gain approval (ongoing or completed) for similar device configurations marketed by various manufacturers. Based on the results from these trials and registries, the Centers for Medicare and Medicaid Services (CMS) approved coverage for CAS with distal embolic protection in high-risk symptomatic patients with stenoses of 70% or greater (Box 47.2). Additionally, coverage was provided for high-risk symptomatic patients with between 50% and 70% stenoses and high-risk asymptomatic patients with 80% or greater stenoses in accordance with category B investigational device exemption (IDE) clinical trials regulation and enrollment in CAS postapproval studies.
Box 47.2 Patients at High Risk for Carotid Endarterectomy
Significant comorbid conditions include but are not limited to:
1. Congestive heart failure class III/IV
2. Left ventricular ejection fraction <30%
4. Contralateral carotid occlusion
5. Recent myocardial infarction
6. Previous CEA with recurrent stenosis
7. Prior radiation treatment to the neck; and
8. Other conditions that were used to determine patients at high risk for CEA in the prior carotid angioplasty followed by stenting (CAS) trials
Supplementing the results generated by the SAPPHIRE trial and subsequent registry studies was the Carotid Revascularization Using Endarterectomy or Stenting Systems (CARESS) trial (CARESS Steering Committee, 2003). This nonrandomized investigation examined all patient subsets (not limited to high risk) and allowed practitioners to determine treatment allocation. In a more realistic clinical paradigm, the study sought to address whether CAS with distal embolic protection was comparable to CEA in patients with symptomatic and asymptomatic carotid stenosis. One-year outcome data demonstrated a trend toward fewer strokes and death in the CAS group, but the difference was not statistically significant (13.6% CEA versus 10% CAS).
Results from two randomized European trials were published simultaneously in 2006. The Stent Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial was a German study that enrolled symptomatic patients with stenoses greater than 50% (by NASCET criteria) and randomly allocated them to CAS or CEA (Ringleb et al., 2006; Stingele et al., 2008). The primary endpoint was ipsilateral stroke or death at 30 days. The steering committee terminated the trial early because of insufficient funding and the large projected number of patients required to demonstrate noninferiority. Nevertheless, primary outcome data appeared to demonstrate near equivalence among the two groups, despite a lack of EPD utilization in the majority of CAS patients.
The French Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial had inclusion criteria similar to SPACE (symptomatic, >60% stenosis) (Mas et al., 2006). Primary outcome included any stroke or death at 30 days. The investigation was terminated prematurely owing to objective concerns for safety and futility. The primary endpoint was achieved in 9.6% of CAS patients and 3.9% of CEA patients (P = 0.01). Detractors claim that the infrequent use of EPDs and deficiencies in the experience of stent operators (as few as five lifetime procedures) may have contributed to poor outcome in the CAS cohort. Nonetheless, the disparate SPACE and EVA-3S results further complicated questions regarding CAS indications in standard-risk symptomatic patients. Furthermore, a secondary analysis of pooled data generated from the SPACE and EVA studies failed to demonstrate that outcome event frequency (stroke or death) was directly related to the use of EPDs, suggesting that not all embolic complications could be avoided with device utilization (Tietke and Jansen, 2009).
The large randomized prospective Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) enrolled a total of 1326 symptomatic patients with greater than 50% stenoses and 1176 asymptomatic patients with greater than 60% stenoses deemed good candidates for either CEA or CAS (Hobson et al., 2004). Primary endpoints included death, stroke, and MI at 30 days or ipsilateral stroke at 1 year. Initial data suggested noninferiority of carotid artery angioplasty and stenting in this broad cohort. Findings indicated that the primary endpoint was not significantly different between the two arms (7.2% versus 6.8%). Additionally, the incidence of death, MI, or stroke at 30 days was similar between the two arms (5.2% versus 4.5%). Periprocedural strokes were significantly higher in the CAS arm (4.1% versus 2.3%), but the incidence of debilitating and major strokes was similar between the two arms (0.9% versus 0.7%). The incidence of MI was significantly lower in the CAS arm, compared with the CEA arm (1.1% versus 2.3%). Cranial nerve palsies were also significantly more common in the CEA arm. Long-term follow-up suggested that the incidence of ipsilateral stroke after the periprocedural period (approximately 4 years of follow-up) was similar between the two arms (2.0% versus 2.4%). Subgroup analyses indicated no difference based on gender, but there seemed to be evidence of effect modification by age such that patients 69 years of age or younger did better with CAS, whereas those aged 70 or older did better with CEA (Brott et al., 2010). These results will likely encourage expansion of indications for CAS procedures.
The International Carotid Stenting Study (ICSS), also known as CAVATAS II, is a European trial in symptomatic carotid stenosis patients. It was designed to revisit the questions raised in the original investigation while using stents in all percutaneous procedures (Ederle et al., 2010). Initial results were published at the same time the CREST data were revealed. ICSS enrolled 1713 patients and demonstrated a higher incidence of ischemic stroke and related events after stenting. The incidence of stroke, death, or procedural MI was significantly elevated in the stenting group (8.5%) compared to that the endarterectomy group (5.2%). Risks of any stroke and all-cause deaths were higher in the stenting group than in the endarterectomy group. There was an increased number of cranial nerve palsies in the CEA group.
Several other trials addressed the indications for CAS in patients who are not high risk for surgery. The ongoing ACT I investigation compared stenting and endarterectomy in standard–surgical risk, asymptomatic patients (Ricotta and Malgor, 2008). The proposed Transatlantic Asymptomatic Carotid Intervention Trial (TACIT) aimed to incorporate an optimal medical management arm comparing the best modern medical treatment regimen to CEA and CAS (Gaines and Randall, 2005; Ricotta and Malgor, 2008). Results generated from investigations such as these will help clarify the future role for CAS procedures. Lessons learned from prior studies will likely translate to rapid development of novel technology and refinement of clinical parameters explored in future CAS trials.
Intracranial Atherosclerotic Disease
Intracranial arterial stenosis is a common etiology of cerebrovascular disease, responsible for at least 9% of all ischemic strokes (Sacco et al., 1995). The prognosis for individuals afflicted with this pathology remains poor owing to a paucity of therapeutic options and a relative inefficiency of medical treatment. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial and subset analyses demonstrated that patients who had suffered an ischemic cerebrovascular event and harbored more than 70% stenosis of a referable intracranial artery were at an exceptionally high risk for recurrent stroke (Chimowitz et al., 2005). A reported 23% of enrolled patients experienced a subsequent ipsilateral stroke over the course of the next year despite aggressive medical therapy (Chimowitz et al., 2005). This high event rate was experienced equally regardless of whether or not patients had failed antithrombotic therapy at the time of their qualifying event (Turan et al., 2009).
Intracranial Angioplasty
Balloon angioplasty was the first percutaneous transluminal technique evaluated. In a large retrospective study, Marks et al. (2006) reported on 120 patients with intracranial stenoses treated by primary angioplasty. The authors calculated a combined periprocedural stroke and death rate of 5.8% and a total yearly event rate of 3.2%. This and other investigations have reported posttreatment residual vessel stenoses in the range of 40% (Marks et al., 2006; Terada et al., 1996). High incidences of angiographic vessel dissection have been documented, although they have rarely been noted to manifest as clinical events (Kiyosue et al., 2004; Marks et al., 2006; Mori et al., 1998; Wojak et al., 2006). Durability of primary angioplasty has also been questioned, with documented retreatment rates near 20% (Siddiq et al., 2008). Mori et al. (1998) investigated lesion-specific features that predicted successful angioplasty and low restenosis rates. They determined that short segment, concentric narrowing, and subtotal angiographic occlusion correlated with the lowest incidence of restenosis following percutaneous transluminal angioplasty (PTA). By contrast, features that portended poor outcome or higher rates of restenosis were eccentricity, extreme angularity, length greater than 10 mm, or excessive tortuosity of the proximal vessel segment. Additionally, the success rate was significantly lower for lesions treated greater than 3 months from the time of stroke.
Experience in the coronary literature indicated that the shortcomings of primary angioplasty (plaque dislodgement, acute elastic recoil, vessel dissection, recurrent stenosis) could be overcome by subsequent stent deployment (George et al., 1998; Savage et al., 1998; Serruys et al., 1994). These concepts were translated directly to the intracranial vasculature.
Intracranial Angioplasty and Stenting
Several early case reports described angioplasty and stenting of cerebral vessels using coronary artery techniques (Chow et al., 2005; Feldman et al., 1996; Gomez et al., 2000; Yu et al., 2005). Device rigidity and vascular tortuosity rendered navigation difficult, resulting in periprocedural morbidity and mortality rates greater than that of angioplasty alone (Chow et al., 2005; Gomez et al., 2000; Jiang et al., 2004; Kim et al., 2004; Levy et al., 2001; Yu et al., 2005). The multicenter nonrandomized Stenting in Symptomatic Atherosclerotic Lesions of Vertebral and Intracranial Arteries (SSYLVIA) trial reported the safety and efficacy of a balloon-mounted stent (Neurolink, Guidant Corporation, Indianapolis, Indiana) designed specifically for craniocervical application; 43 patients with symptomatic intracranial disease and 18 with extracranial vertebral artery stenoses were evaluated. Successful stent placement was achieved in 95% of patients. The investigators reported a 6.6% periprocedural stroke rate and an additional 7.3% incidence of stroke at 1-year follow-up. However, angiographic restenosis was documented in 35% of patients and was symptomatic in over one-third of these individuals (SSYLVIA Study Investigators, 2004). Based on these data, the FDA granted the stent a humanitarian device exemption (HDE) for the treatment of high-risk patients with significant intracranial and extracranial atherosclerotic disease who had failed medical therapy. More recent studies have investigated modified balloon-mounted stenting systems designed exclusively for the cerebral vasculature. Jiang et al. (2004) documented a 91.7% rate of technical success with the Apollo intracranial stent (MicroPort Medical, Shanghai, China) in patients harboring stenoses greater than 50%. The primary endpoint, ischemic stroke in the target lesion arterial territory or any stroke/death within 30 days, occurred at a rate of 4.3 per 100 patient-years. A restenosis rate of 28% was reported. The Vitesse Intracranial Stent Study for Ischemic Therapy (VISSIT), designed to evaluate the Pharos Vitesse system (Micrus Endovascular, San Jose, California), is currently underway (Kurre et al., 2008b).
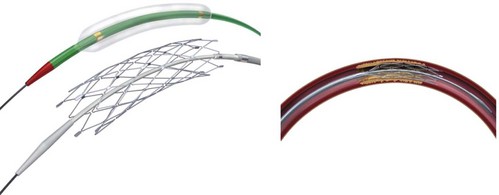
The Wingspan stent is the first self-expanding system designed solely for intracranial application (Fig. 47.8). A nitinol (nickel and titanium metal alloy with shape memory and superelasticity) construct with high radial force allows navigation of small and tortuous intracranial vessels. Recently the FDA granted an HDE approval for the Wingspan Stent System with Gateway PTA balloon catheter (Boston Scientific, Natick, Massachusetts) for symptomatic ICAD referable to an intracranial vessel with greater than 50% stenosis and refractory to medical therapy. Endorsement was predicated on a safety study conducted in Europe and Asia (Bose et al., 2007). In that initial investigation, technical success was achieved in 98% of patients, and the 6-month death or ipsilateral stroke rate was 7%, with an all-cause stoke rate of 9.7%. In the more recent NIH Multicenter Wingspan Intracranial Stent Registry Study, 129 patients with symptomatic 70% to 99% intracranial stenoses were enrolled. The technical success rate was 97%. The frequency of any stroke, intracerebral hemorrhage, and death within 30 days or ipsilateral stroke beyond 30 days was 14% at 6 months (Zaidat et al., 2008). The reported frequency of angiographic restenosis was 25%.
In-stent restenosis has decreased following cardiac angioplasty and stenting procedures with the advent of drug-eluting stents (Regar et al., 2002). While this generation of stents may be useful in reducing myointimal hyperplasia, no platforms have been specifically designed and approved for use in the small and tortuous intracranial vessels.
Angioplasty/Stenting Procedure
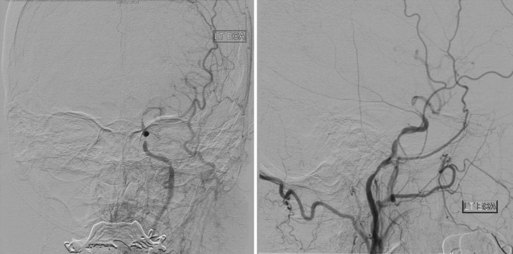
Intracranial angioplasty and stenting with the Gateway/Wingspan system is most often performed under general endotracheal anesthesia. Dual antiplatelet therapy is initiated in anticipation of the procedure. A diagnostic cerebral angiogram is performed to assess degree of stenosis, length of diseased segment, flow restriction, and the presence of leptomeningeal collaterals. A shuttle sheath or guide catheter is used to maintain proximal position and accommodate the angioplasty/stenting platform. Some operators use a distal support catheter in patients with particularly tortuous anatomy. After a microcatheter/microguidewire system is advanced past the lesion, an exchange-length microwire is used to advance, in series, the balloon angioplasty and stent catheters to the level of stenosis. The lesion is initially dilated with a PTA balloon sized to 80% of the native vessel luminal diameter, using the slow inflation technique advocated by Connors and Wojak (Connors and Wojak, 1999). After initial vessel dilation, the balloon catheter is exchanged for the self-expanding stent system, which is deployed across the stenotic lesion. Post-stenting balloon dilation is discouraged by the manufacturer (Figs. 47.9 and 47.10). At the conclusion of the procedure, the average reported residual stenosis (30%) is less than that observed following PTA alone (40%) but more than noted after balloon-mounted stent deployment (10%) (Fiorella and Woo, 2007; Henkes et al., 2005). Follow-up angiograms have demonstrated restenosis rates exceeding 30%, presumably due to elastic recoil, myointimal hyperplasia, and vascular remodeling (Garas et al., 2001). Coating stents with antiproliferative agents such as sirolimus and paclitaxel has been effective in reducing restenosis rates in the cardiology literature (Ong et al., 2006; Stone et al., 2004). Several recent studies have demonstrated encouraging results for the treatment of ICAD patients with drug-eluting stents (Gupta et al., 2006; Qureshi et al., 2006). Difficult vessel navigation with more rigid stenting systems and prolonged postoperative treatment with dual antiplatelet therapies are among the potential limitations to the successful translation of drug-eluting technology to pathologies of the intracranial vasculature.
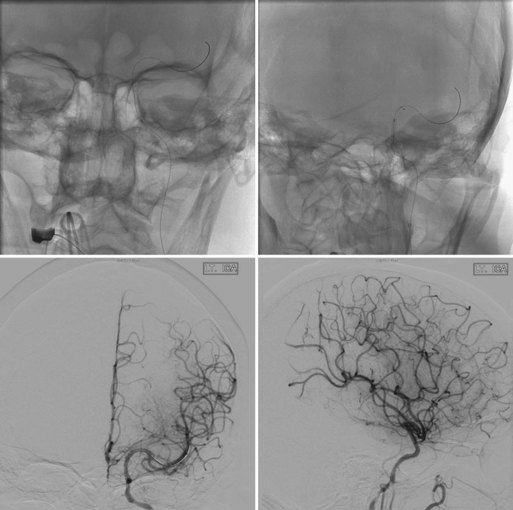
Fig. 47.10 Same patient as in Fig. 47.9. Top x-rays demonstrate balloon positioned across petrocavernous stenosis (black marker is the midpoint of the balloon, left) and stents deployed across petrocavernous and supraclinoid lesions (right). Anteroposterior (left) and lateral (right) post-stenting angiograms on the bottom demonstrate increased flow through stenoses and filling of both anterior and middle cerebral arteries on left.
Clinical Application
There remains controversy as to whether intracranial stent placement is superior to primary angioplasty in the treatment of ICAD. Studies have addressed the angiographic and clinical outcomes associated with each procedure, but relatively few comparative investigations have been reported. A multi-institutional (three tertiary care centers) retrospective study compared the clinical outcomes between primary angioplasty and stent placement for symptomatic intracranial atherosclerosis (Siddiq et al., 2008, 2009). Stroke and combined stroke and/or death were identified as primary clinical endpoints during the periprocedural and follow-up period of 5 years. The authors noted significantly fewer residual stenoses in the stent-treated cohort, but there was no significant reduction in stroke or mortality among the patients who underwent stenting procedures.
In a systematic literature review and meta-analysis, Siddiq et al. (2009) compared primary angioplasty and stent placement in patients with symptomatic intracranial atherosclerosis. They noted technical success rates of 80% in the angioplasty group and 95% in the stent cohort. There was no difference in the perioperative stroke and death rate between the two treatment groups (9% angioplasty, 8% stent). The pooled incidence of 1-year stroke and death was 20% in patients treated with primary angioplasty and 14% in those treated with stenting, signifying a statistically significant difference in outcome. Also noted was a significantly higher restenosis rate in the primary angioplasty group. Importantly, the authors demonstrated no discernable effect of study publication year on the risk of stroke and death.
Debate remains as to the precise indications and preferred treatment strategies for symptomatic ICAD. The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study is an ongoing large-scale randomized investigation comparing intracranial angioplasty and stenting (Wingspan stent/Gateway balloon) and intensive medical management with optimal medical management alone. Patients with 70% to 99% intracranial stenosis who suffered a stroke or transient ischemic attack (TIA) within 30 days of enrollment are randomized and followed for up to 4 years. This marks the first large-scale randomized study comparing the efficacy of endovascular therapy and medical management in patients with ICAD (Rasmussen, 2009). Intracranial angioplasty with or without stenting is an emerging technique that may have a pivotal position in the treatment of symptomatic intracranial atherosclerosis. The results of SAMMPRIS and other ongoing investigations will better address the precise indications.
Hemorrhagic Stroke
Cerebral Aneurysms
Intracranial aneurysms are abnormal focal dilatations of the cerebral arteries, with thinning and weakening of the vessel wall. Aneurysms constitute a common cerebrovascular pathology, with a reported adult prevalence of 0.2% to 9% worldwide (Inagawa and Hirano, 1990). Vessel degeneration and hemodynamic factors contribute to changes in focal sheer stress and flow patterns that ultimately may result in aneurysmal enlargement or rupture.
Most unruptured aneurysms remain undetected, but an increasing number of incidental aneurysms is being discovered during diagnostic imaging studies (Wakhloo, 2008; Wiebers et al., 2003). Although patients harboring intracranial aneurysms may present with symptoms resulting from mass effect or thromboembolism, the most common and perilous clinical manifestation is that of subarachnoid hemorrhage (SAH). It is estimated that 10 of every 100,000 intracranial aneurysms rupture annually. Aneurysmal rupture carries a roughly 50% mortality rate, with 10% to 20% of survivors failing to regain long-term functional independence (Ellegala and Day, 2005; Hop et al., 1997; Schievink, 1997).
Decision Matrix
Ruptured Aneurysms
Following rupture of an intracranial aneurysm, recurrent hemorrhage occurs at a high frequency. Jane et al. (1985) documented that the incidence of rebleeding among patients with SAH and untreated aneurysms was 50% in the first 6 months and 3% per year thereafter. Because of the high risk of subsequent rupture, these aneurysms are nearly always treated urgently.
The randomized prospective multicenter International Subarachnoid Aneurysm Trial compared the efficacy of endovascular treatment with that of surgery in patients with ruptured intracranial aneurysms suitable for either treatment modality (Molyneux et al., 2002, 2005). A total of 2143 patients were randomized. Recruitment was prematurely terminated after a planned interim analysis demonstrated reduced disability in the endovascular treatment group. In an intention-to-treat analysis, the proportion of patients who were dead or disabled at 1 year was significantly lower in the endovascular therapy group compared to the surgical cohort. Additionally, the risk of perioperative seizures was lower following coiling procedures. However, the requirement for a second procedure was higher with endovascular treatment. The 1-year risk of rebleeding from the ruptured aneurysm was 2 per 1272 patient-years for individuals allocated to embolization and 0 per 1081 in the cohort allocated to open surgery. The investigators noted that the low rates of late rebleeding and potential periprocedural complications associated with retreatment in the endovascular group were unlikely to alter the treatment benefit at 1 year. They concluded that endovascular coil embolization of ruptured intracranial aneurysms when a patient has a good clinical grade (World Federation of Neurosurgical Societies grade I-III) and the aneurysm anatomy is suitable for endovascular treatment is more likely than not to lead to independent survival at 1 year those receiving neurosurgical treatment. Results may be generalized to good-grade patients harboring anterior circulation aneurysms. Statistical comparisons were difficult in poor-grade patients and those with posterior circulation aneurysms, as the vast majority of these individuals were not randomized due to treating physicians’ overwhelming preference for endovascular therapy.
To address issues regarding incomplete occlusion and recanalization following endovascular therapy, rerupture rates after treatment for aneurysmal subarachnoid hemorrhage were further investigated in the Cerebral Aneurysm Rupture After Treatment (CARAT) study (Johnston et al., 2008). All ruptured saccular aneurysms treated between 1996 and 1998 in nine institutions were evaluated for rerupture. A total of 1010 patients (711 surgically clipped, 299 treated by coil embolization) were contacted by written questionnaire or telephone. Rerupture of the treated aneurysm after 1 year occurred in one patient who underwent coil embolization (904 person-years of follow-up, 0.11% annual rate) and no patients treated with surgical clipping (2666 person-years). Aneurysm retreatment after 1 year was more frequent in the endovascular cohort. However, major complications were rare during follow-up treatment. The authors concluded that late events are unlikely to overwhelm differences between the procedures at 1-year follow-up.
Incidental Aneurysms
Rupture rates for intracranial aneurysms vary with size and location, as reported by the International Study of Unruptured Intracranial Aneurysms (International Study of Unruptured Intracranial Aneurysms Investigators, 1998; Wiebers et al., 2003). The prospective observational arm of this large study investigated 2686 unruptured, untreated cerebral aneurysms in 1692 patients. Individuals with no history of SAH who harbor incidental aneurysms located in the anterior circulation (ICA, anterior communicating or anterior cerebral artery, middle cerebral artery) had 5-year cumulative rupture rates of 0%, 2.6%, 14.5%, and 40% for aneurysms of less than 7 mm, 7 to 12 mm, 13 to 24 mm, and 25 mm or greater, respectively. Rupture rates for aneurysms of the same sizes located at the posterior communicating artery or in the posterior circulation were 2.5%, 14.5%, 18.4%, and 50%, respectively. Patients with a prior history of SAH resulting from rupture of an aneurysm distinct from that being studied had a 5-year cumulative rupture rate of 1.5% for aneurysms less than 7 mm in the anterior circulation, compared with 3.4% for aneurysms of the same size located at the posterior communicating artery or in the posterior circulation. Aneurysms greater than 7 mm in this cohort had similar rupture rates to the group with no prior history of SAH (Wiebers et al., 2003).
Therefore, it is generally believed that incidental aneurysms less than 7 mm, located in the anterior circulation, and in patients with no prior history of SAH may be managed expectantly with serial imaging. However, patient-specific desires or the presence of factors such as irregular morphology, documented growth, or a family history of intracranial aneurysms may encourage consideration of surgical or endovascular treatment even in this low-risk cohort (Broderick et al., 2009). Recent studies have focused on quantitative hemodynamic analysis using computer-generated flow models to predict aneurysmal growth and rupture (Chien, 2009). While these methods hold great promise, further development and application to large patient cohorts is required to develop reliable algorithms that accurately predict rupture risk in the clinical setting. According to the ISUIA data, aneurysms of any size located in the posterior circulation and those in patients who have experienced prior SAH carry a significant 5-year cumulative rupture risk and warrant treatment consideration (Wiebers et al., 2003).
There exist no large randomized controlled trials favoring endovascular or surgical management of unruptured intracranial aneurysms. Individualized algorithms must be tailored to specific clinical and anatomical factors when selecting an appropriate treatment paradigm (Raja et al., 2008).
Several large retrospective studies document very low morbidity and mortality rates for surgical clipping in the hands of experienced neurosurgeons (Bederson et al., 2000; Deruty et al., 1996; Dickey et al., 1994; Komotar et al., 2008a; Moroi et al., 2005; Raaymakers et al., 1998). Many authors promote microsurgical treatment in young patients with small anterior circulation aneurysms, suggesting that surgical clipping provides a more durable repair than coil embolization (Komotar et al., 2008a).
Observational studies have advocated surgical clipping for broad-necked aneurysms (Ogilvy et al., 2002; Raftopoulos et al., 2000, 2003; Regli et al., 1999, 2002) and for those with critical vessels emanating from the base (Johnston et al., 2001; Raftopoulos et al., 2000; Regli et al., 1999, 2002).
In 2003, Ogilvy and Carter retrospectively reviewed a series of 604 unruptured aneurysms in an attempt to identify risk factors associated with outcome following surgical treatment. The authors found patient age, aneurysm size, and location within the posterior circulation to be independent predictors of poor outcome (Ogilvy and Carter, 2003).
Emerging data on the endovascular treatment of unruptured intracranial aneurysms suggest low morbidity and mortality rates. Large studies indicate an overall morbidity of 5% to 10%, with mortality rates very close to zero (Johnston et al., 2001; Pouratian et al., 2006; Wiebers et al., 2003). Johnston et al. (2001) compared the safety of endovascular coiling and microsurgical clipping in 2069 patients treated in California between 1990 and 1998. In-hospital mortality and discharge to an alternate facility (rather than home) were significantly less frequent in the endovascular cohort. Mortality was 0.5% in the endovascular group and 3.5% in the open surgical cohort. These results are striking given that the investigation was conducted during the relatively early development of endovascular coiling technology. More recent data from the ISUIA study suggest that endovascular morbidity and mortality may be lower at 1 year than that reported for open surgical treatment in both patients with (7.1% versus 10.1%) and without (9.8% versus 12.6%) prior SAH (Wiebers et al., 2003). Furthermore, outcome following endovascular procedures appeared to be less dependent upon patient age. Direct comparisons were not generated, however, because the endovascular cohort was relatively small. Physician preference led to coil embolization treatment of a greater percentage of older medically ill patients and those with aneurysms located in the posterior circulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree