Fig. 11.1
Prolactinoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A small, hypoenhancing sellar mass is present in the left aspect of the sella. The mass abuts the left cavernous internal carotid artery without encasement
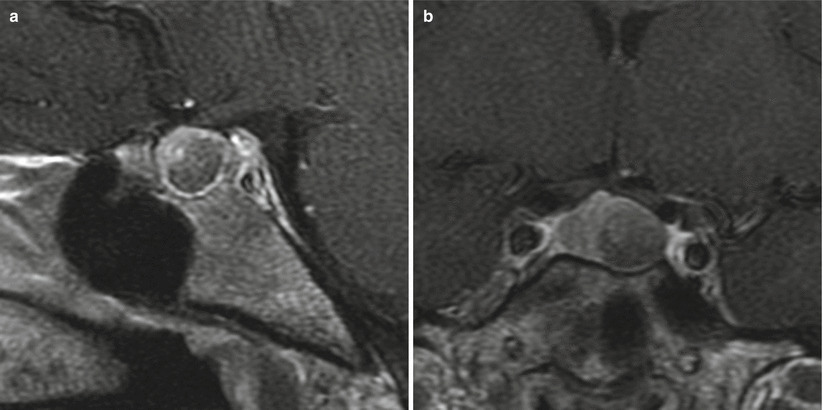
Fig. 11.2
Prolactinoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is a hypoenhancing sellar mass in the left aspect of the sella, with erosion of the left sellar floor. The mass abuts the left cavernous internal carotid artery without encasement. There is mild elevation and rightward deviation of the pituitary stalk
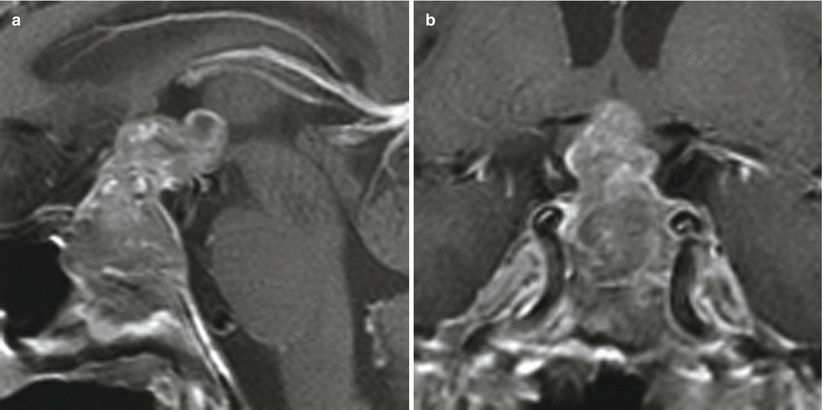
Fig. 11.3
Prolactinoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A heterogeneously enhancing sellar mass shows suprasellar extension and erosion of the sellar floor. The irregular, nodular suprasellar component extends to the floor of the third ventricle, contacting the hypothalamus. The mass abuts the cavernous internal carotid arteries without encasement
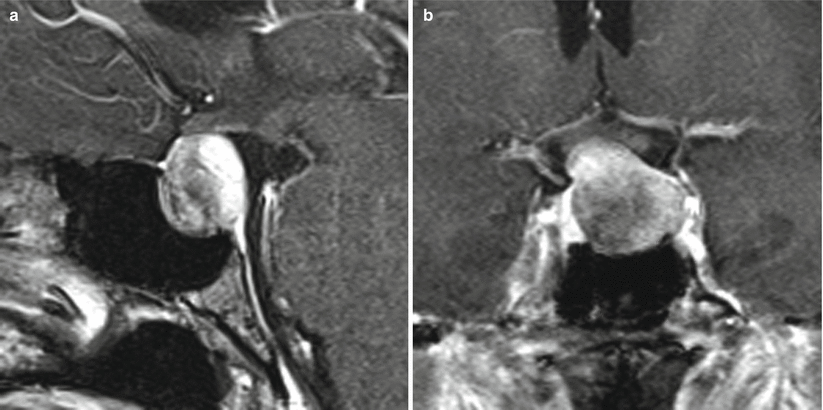
Fig. 11.4
Prolactinoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is a hypoenhancing sellar mass with suprasellar extension and remodeling of the sellar floor. The mass abuts the left cavernous internal carotid artery without encasement. The mass also contacts the optic chiasm without significant displacement
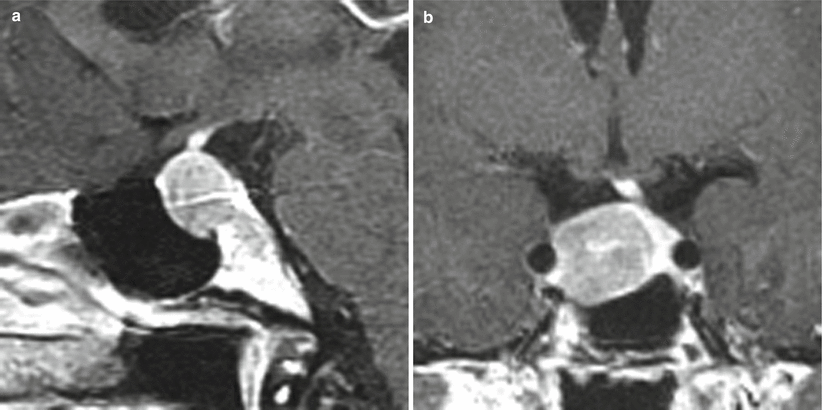
Fig. 11.5
Prolactinoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A hypoenhancing sellar mass is located in the right aspect of the sella, with erosion of the left sellar floor. The mass abuts the right cavernous internal carotid artery without encasement. There is leftward deviation of the pituitary stalk
11.2.2 Histopathology
Figure 11.6 shows several histopathologic characteristics of prolactin (PRL) cell adenomas, including central nuclei with delicate chromatin, microcalcifications, and focal PRL immunoreactivity in the tumor cells despite treatment.
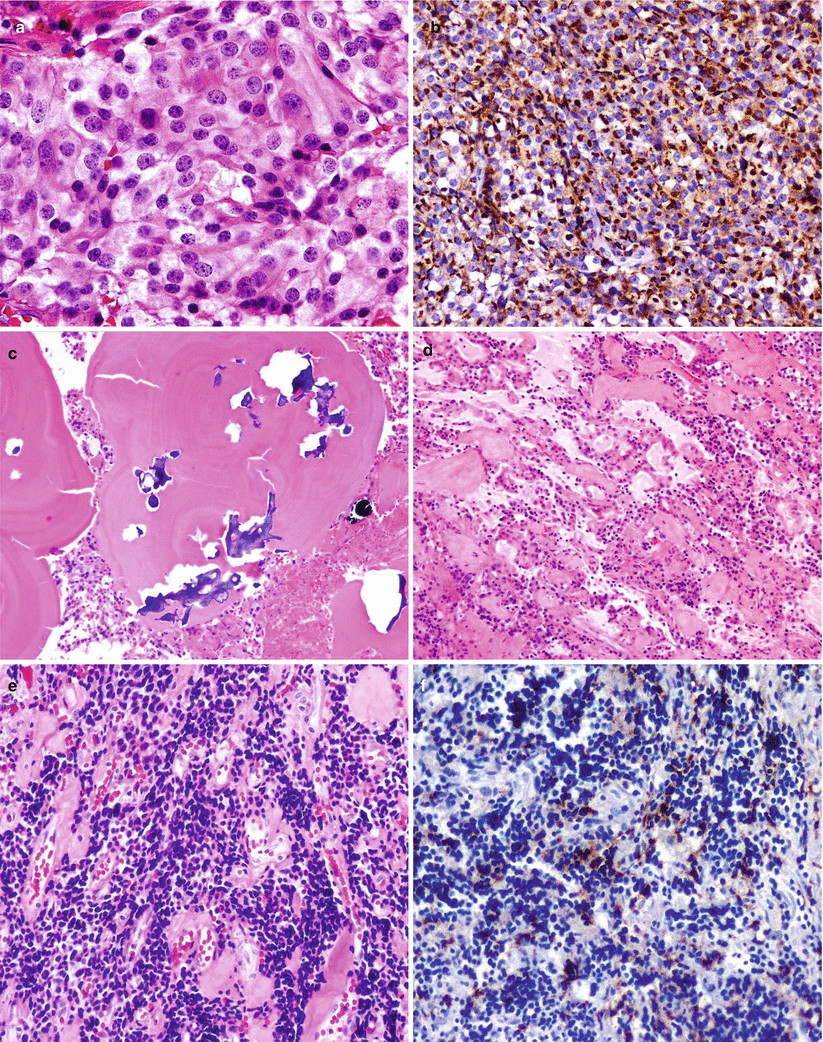
Fig. 11.6
Prolactin (PRL)-secreting adenoma. (a) PRL cell adenoma showing chromophobic-to-acidophilic cytoplasm and central nuclei with delicate chromatin. (b) PRL immunoreactivity is typically seen in a paranuclear location. (c) Microcalcifications are commonly seen in these tumors. (d, e) Prolactinomas may display variable degrees of shrinkage of the tumor cells and interstitial fibrosis after medical treatment. (f) Despite treatment, PRL immunoreactivity is focally seen in the tumor cells
11.3 Clinical Management
11.3.1 Endocrinological Diagnosis
A serum prolactin level greater than 200 ng/mL is typically indicative of a prolactin-secreting adenoma [5].
Moderately elevated levels (<200 ng/mL) require correlation to the size of the tumor, as they may represent a stalk compression effect rather than a prolactinoma.
Microprolactinomas also may have prolactin levels in the range of 30–200 ng/mL [1]. It should also be noted that various physiological states (e.g., pregnancy and breastfeeding) and a wide variety of medications (e.g., antipsychotics) may elevate the serum prolactin level.
Clinicians must beware of the “hook effect” phenomenon, in which an excessively high serum prolactin level results in a laboratory value that is significantly lower than the actual value, secondary to the presence of excessive antigen [6]. This effect can be avoided if serial dilution testing of serum samples is carried out for all macroadenomas that are suspected of being prolactinomas.
Macroprolactinemia is another diagnostic pitfall, in which macroprolactin, a biologically inactive form of prolactin with a high molecular mass, is detected by standard immunoassays. The presence of macroprolactin may lead to misdiagnosis in as many as 10 % of all reported instances of biochemical hyperprolactinemia, so it should be included in the differential diagnosis of hyperprolactinemia [7]. Gel filtration chromatography remains the gold standard for diagnosing macroprolactinemia.
11.3.2 Medical Treatment
Prolactinomas usually can be treated successfully with medical management.
Dopamine agonists (DAs) are the first line of therapy, with bromocriptine and cabergoline being the two most commonly used agents.
Cabergoline is more potent than bromocriptine and therefore requires dosing less frequently (usually twice a week).
DAs are effective in reducing tumor size in the majority of patients; tumors may shrink up to 80 % within 6 weeks of medication use [9].
Successful treatment accompanied by rapid tumor shrinkage has been reported to result in cerebrospinal fluid leaks, however, which often require surgical repair [10].
For women desiring to become pregnant, bromocriptine therapy may be initiated and then discontinued once the patient is known to be pregnant [11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








