Fig. 3.1
Marker expression of primary meningioma cell cultures. Positive immunoreactivity appears as green staining (Alexa Fluor 488). (a) vimentin; (b) CD44; (c) EMA. Original magnification 20×
3.1.2 Immunocytochemical Staining
An immunocytochemical analysis was performed on meningioma cells growing in a monolayer fashion in culture. The cells were plated in 4-well glass slides and allowed to remain in growth media for 24 h. After removal of the growth media, the cells were rinsed with PBS for 5 min followed by fixation with ice-cold methanol for 10 min at – 20 °C. The slides were rinsed with PBS and incubated with 3 % H2O2 for 10 min at RT. All cells were blocked with 10 % rabbit serum in PBS for 30 min at room temperature. Slides were incubated with primary antibodies overnight at 4 °C. Staining was developed using the VECTASTAIN Elite ABC system and Vector® NovaRED™ (Vector Laboratories, Burlingame, CA). Sections were counterstained with haematoxylin and examined by light microscopic studies (Fig. 3.2).
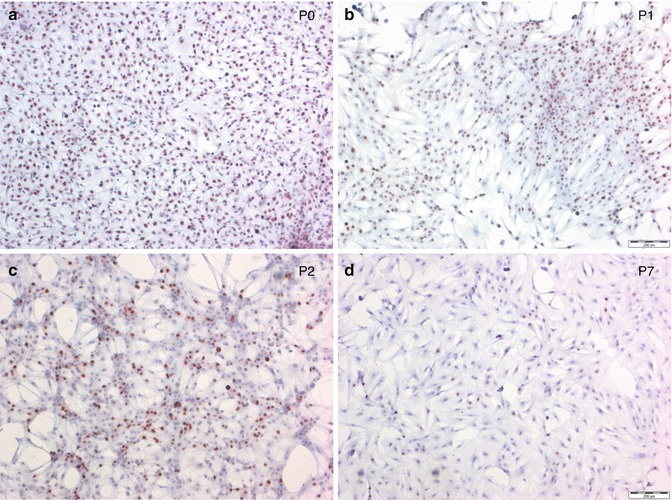

Fig. 3.2
Proliferation rate in primary meningioma cell cultures decreases with increasing passage (P). (a–d) Ki-67 nuclear staining in a representative meningioma. Positive immunoreactivity appears as red staining (Vector® NovaRED™). Original magnification 4×. (a) P0; (b) P1; (c) P2; and (d) P7
3.1.3 Senescence-Associated β-Galactosidase Staining
Expression of senescent-associated β-galactosidase activity was analysed in different passages of primary meningioma cell cultures using the Cellular Senescence Detection Kit (BioVision, Milpitas, CA) (Fig. 3.3).
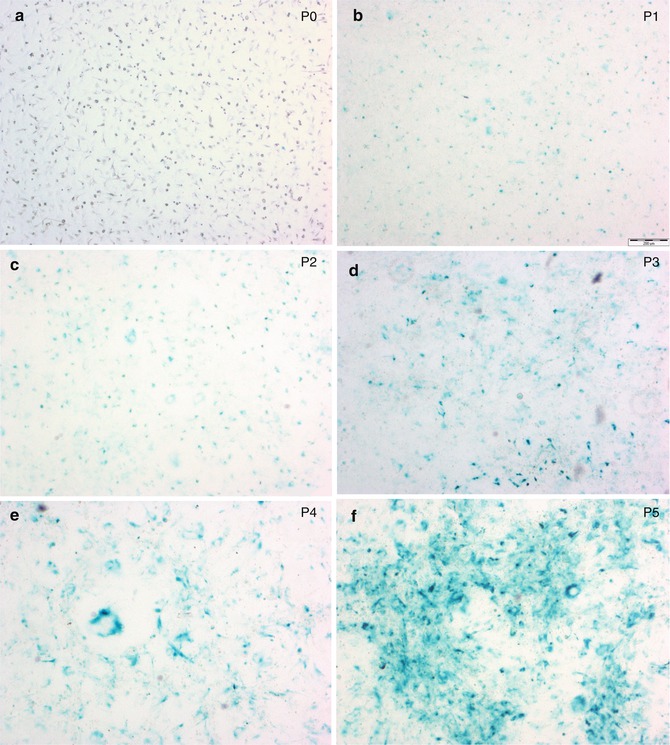

Fig. 3.3
Senescence development in primary meningioma cell cultures. Senescent cells express a senescent-associated β-galactosidase (X-gal). X-gal positive cells appear as blue-green staining. Original magnification 4×. (a) P0; (b) P1; (c) P2; (d) P3; (e) P4; and (f) P5
3.2 Results
The mean Mib-1 SI in male patients was 5.8 %, whereas that of female patients was 3.9 %; patients with NF-II show significant higher mean Ki-67 LI (6.2 %). Ki-67 LI in WHO I tumours was significantly lower than in WHO II (atypical) and WHO III (anaplastic) (p < 0.0001), but not within the subclassifications of WHO grading (meningotheliomatous 3.3 %, fibrous 3.9 %, transitional 2.9 %, psammomatous 1.1 %, angiomatous 3.0 %, clear cell 0.7 % or microcystic meningiomas 4.4 %) (Table 3.1). First time treated meningiomas had a mean Ki-67 LI of 3.9 % versus 6.9 % in recurrent meningiomas (P < 0.0001). Ki-67 LI increases from the initial tumour operation to its second, third and in 5 cases to its fourth local recurrence. There was a transformation from benign to atypical meningioma from second to third local recurrence in 16 % of the cases, whereas no dedifferentiation from WHO I to WHO III or WHO II to WHO III has been seen. Significantly lower PR expression in WHO II (atypical) and III (anaplastic) meningiomas, compared to WHO I meningiomas, has also been observed.
Table 3.1
WHO grading in 1766 meningiomas operated from 1979 to 2002
WHO grading | Young (<70 years) | Aged (≥70 years) | ||
|---|---|---|---|---|
n = 1,544 | n = 222 | |||
Female | Male | Female | Male | |
n = 1,088 | n = 456 | n = 162 | n = 60 | |
I | 92.2 % (1,424) | 91.9 % (204) | ||
Meningotheliomatous | 63.8 % (694) | 58.6 % (267) | 54.9 % (89) | 58.3 % (35) |
Fibrous | 13.1 % (142) | 11.2 % (51) | 13.6 % (22) | 8.3 % (5) |
Transitional | 11.0 % (120) | 8.1 % (37) | 11.1 % (18) | 8.3 % (5) |
Psammomatous | 5.2 % (57) | 2.6 % (12) | 11.1 % (18) | 10.0 % (6) |
Other types | 2.2 % (24) | 4.4 % (20) | 1.9 % (3) | 5.0 % (3) |
II | 6.4 % (99) | 7.2 % (16) | ||
Atypical (including clear cell, secretory) | 4.0 % (43) | 12.3 % (56) | 6.8 % (11) | 8.3 % (5) |
III | 1.4 % (21) | 0.9 % (2) | ||
Anaplastic | 0.7 % (8) | 2.9 % (13) | 0.6 % (1) | 1.7 % (1) |
Excluding WHO II and III meningiomas, as well as partially resected meningiomas for survival analysis, in WHO I and SI resected meningiomas, no significant difference in survival according to different mean Ki-67 LI or PR status has been found. Combining two factors of proposed prognostic significance in benign meningiomas – proliferation index (Ki-67 LI < 4 % vs. ≥ 4 %) and PR status (neg. vs. pos.) – a significant decreased recurrence-free survival could be shown for negative PR status and Ki-67 LI ≥ 4 % (Fig. 3.4).
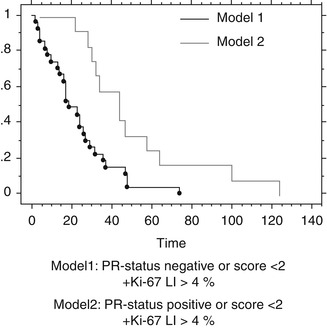

Fig. 3.4
Recurrence-free survival II. Kaplan-Meyer Cum. Hazard plot for new recurrence. A model of two prognostic factors considering prognosis in total removed meningiomas (Simpson I + II). Time scale in month
For proliferation and PR status analysis, multiple age groups for each sex have been examined as well (<40, 41–55, 56–70 and >70), but no significance has been seen (Fig. 3.5). No relationship between the calcifications, meningioma subtype or proliferation index could be established. Although recurrent meningiomas exhibit higher proliferation rates in general, no age dependency has been seen.
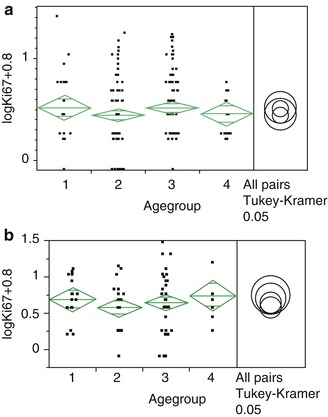

Fig. 3.5
Statistical analysis of Ki-67 distribution in different age groups (1, <40; 2, 41–55; 3, 56–70; 4, >70) and separated for sex (left, females; right, males)
3.3 Discussion
Meningiomas are mostly benign tumours that usually do not invade the brain parenchyma. The WHO grading system aiming to describe different types of tumour frequently fails to determine the clinical behaviour of meningiomas. Even in cases of complete removal according to the Simpson classification the chance of recurrence is high [52]. Pathological specimens in those cases do not show either atypical features or histopathological signs of increased biological activity. Proliferation markers like BrdU or biochemical markers like telomerase and topoisomerase IIα have been used to describe the clinical course in meningioma patients [8, 51]. In the case of BrdU Gudjonssona et al. a PET study showed that BrdU is not suitable as proliferation marker [14]. Whereas telomerase is a potential predictor for recurrence in meningiomas [18, 30], there are conflicting results for topoisomerase IIα as predictor for recurrence in meningiomas [23, 24].
The Mib-1 monoclonal antibody, staining the Ki-67 antigen, has demonstrated its reliability in analysing meningioma growth and recurrence by several authors [1, 33, 36, 39, 47, 48].
Møller separated different resection grades and histology before survival analysis in 25 cases and could not give any significant prediction with Ki-67 immunohistochemistry as well [35]. Abramovich reported the absence of a significant difference in proliferation in 59 meningioma patients that had been radically operated, recognizing a clear overlap of the index range between the groups [1]. On the contrary, Ohta et al. showed a correlation with recurrence-free interval in 42 patients, but the follow-up time was 60 months and the statistics were performed without dividing the meningiomas according to the WHO classification [38]. Perry et al. showed values for interpretation of borderline atypical meningiomas in 425 meningiomas through multivariate analysis [39]. Brain invasion, mitosis count > 3/10 and a mean Ki-67 LI > 4.2 % correlated with decreased recurrence-free survival. Matsuno et al. and Nakasu et al. reported a 3.2 and 3 % cut-off point for higher recurrence tendency [33, 36]. However, 20–50 % of the recurrent meningioma groups were atypical, and all nonrecurrent meningiomas were WHO I. Survival analysis was performed taking all patients together without dividing for surgical resection or histological grade. The small number of patients in each group and the uneven distribution of histological grades might have additionally influenced the mean LI and the survival analysis. Failing to separate different histopathological and resection grades exposes these studies to the criticism that Ki-67 may simply describe a well-known phenomenon, in other words that more aggressive histology and less aggressive regression correlate with shorter disease-free survival times.
Elevated proliferative activity in recurrent meningiomas throughout the histological grouping has been shown [1, 33, 35, 36, 38]. A decrease of the LI has only been reported by Madsen et al. [29]. Caution is recommended when using the proliferation index as the sole prognostic indicator or as a substitute for morphological diagnosis due to the overlap in each group (WHO I mean Ki-67 LI 0–30 %, WHO II mean Ki-67 LI 3–35 % or WHO III mean Ki-67 LI 5–58 %) [19, 22, 27]. This might be due to the heterogeneity of biological activity within the tumour tissue. Proliferating cells in all histological grades were found to be distributed heterogeneously throughout the tumour, especially in recurrences [38]. It is debatable that the focal accumulation of proliferation may affect tumour recurrence for the ‘highest-area’ counting method. The ‘highest-area’ counting method is preferred to minimize missing of focal accumulation of biological activity within the meningioma tissue, as it is recommended by Nakasu et al. [36]. This is an important issue when considering modern neurosurgical techniques, where only small parts of tumour with unknown precise location of the tissue reach the pathologist’s hands. Indeed, it is debatable whether recognized areas of high mitotic activity in meningiomas determined with either counting method reflect the proliferation status of the whole tumour.
A substantial number of patients inherit mean Ki-67 LI of 1 % with recurrence times ranging from 11 to 148 month. Therefore, a cut-off value over which a tumour becomes suspicious cannot be given. Precise values from different laboratories are not applicable to other institutions because of differences in methodology, counting procedures and interpretation of the results as reflected by the relatively wide range of initial and recurrent Ki-67 LI determined by several investigators.
The role of steroid hormones in the progression of meningiomas is still a matter of controversy. Several studies, which included meningiomas of all grades, suggested a more favourable prognosis for PR-positive meningiomas [15, 16] with high progesterone receptor status being associated with lower recurrence rates and vice versa [9, 46]. However, these studies frequently included meningiomas with different resection grades and histological subtypes. Hsu and colleagues suggested that only a combination of three proposed prognostic factors for survival, that is, WHO grade, proliferative index and PR status, should be used to predict meningioma recurrence [16]. However, their model was tested containing atypical and malignant meningiomas and therefore did not demonstrate the true influence of PR status on survival. The focus of interest should be benign borderline cases where additional information is needed for prognostic considerations. Maiuri et al. compared two groups of completely resected WHO I meningiomas, with and without recurrence [31]. Patient age, tumour location, consistency, vascularity, histological subtype, oestrogen receptor, vascular endothelial growth factor (VEGF), and epidermal growth factor receptor (EGFR) were not correlated with tumour recurrence. Higher mitotic index and Ki-67 LI and PR negativity were predictive factors of recurrence of benign completely resected meningiomas. This study also showed a negative association between Bcl-2 and PR.
Data in the literature is pointing to a female preponderance in PR expression; however, these studies included a substantial number of atypical and malignant meningiomas [5, 9, 20]. These meningiomas are mostly devoid of PRs and more often found among male patients. After excluding atypical and malignant cases, no sex-related difference in PR expression could be found. Therefore, the previously reported sex-related difference might be due to particular selection criteria, which produced an inhomogeneous patient population.
Previous studies on the human endometrium have shown that progesterone plays a role in neovasculogenesis. High levels of progesterone receptors in meningiomas may indicate a similar situation. However, Fewings and Roser suggested that progesterone may inhibit angiogenesis, by enhancing the production of thrombospondin-1, an angiogenesis inhibitor [9, 46]. It is difficult to draw conclusions from these data, as the PRs in meningiomas are non-functional in the majority of cases [4]. Moreover, our observations come from a clinical and histopathological analysis and may well reflect a multifactorial situation with progesterone being only one variable in the process of tumour vascularization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








