Chapter 95 REM Sleep Parasomnias
Abstract
Rapid eye movement (REM) sleep is characterized by numerous physiologic variables that usually occur in concert to produce the fully declared REM sleep. The majority of the REM sleep parasomnias reflect state dissociation, a condition seen when not all of the elements usually comprising REM sleep are present, resulting in fascinating clinical phenomena. The most common and best-studied REM sleep parasomnia is the REM sleep behavior disorder (RBD). In patients with RBD, somatic muscle atonia—one of the defining features of REM sleep—is absent, permitting the acting out of dream mentation, often with violent or injurious results. RBD is overwhelmingly a disorder affecting older men, many of whom eventually develop neurodegenerative disorders. Increasingly, medications prescribed for psychiatric symptoms (predominantly selective serotonin reuptake inhibitors), are the cause of RBD. The vast majority of patients with RBD respond to clonazepam. Further study of the REM parasomnias will serve to teach us much about sleep and the function of the central nervous system.1,2
Rem Sleep Behavior Disorder
In experiments reported in 1965, bilateral lesions of pontine regions adjacent to the locus coeruleus in cats caused absence of the expected atonia associated with REM sleep, allowing the cats to demonstrate prominent motor activities during REM sleep (oneiric activities).3 This animal model has been important in further studies demonstrating the similarity between the states of wakefulness and REM sleep4 and in studies evaluating the state-dependent vulnerability to epileptic activity.5 In the 1970s, scattered reports of dream-enacting behavior involving humans appeared; the polygraphic and behavioral condition was sometimes referred to as “stage 1 REM with tonic electromyogram.”6–9 Recognition of REM sleep behavior disorder (RBD) as a distinct clinical disorder followed the report in 1986 of a series of adults with RBD.10 The cat model has been extended to the rat.11,12
Numerous physiologic phenomena occur during REM sleep13–26 and fall into two categories: tonic (appearing throughout a REM period) and phasic (occurring intermittently during a REM period). Tonic elements include electromyographic (EMG) suppression, low-voltage desynchronized electroencephalogram (EEG), high arousal threshold, hippocampal theta rhythm, elevated brain temperature, poikilothermia, olfactory bulb activity, and penile tumescence. Phasic elements include rapid eye movements (REMs), middle ear muscle activity, tongue movements, somatic muscle twitches of the limbs, variability of autonomic activity (cardiac and respiratory), and ponto-geniculo-occipital (PGO) spikes. It is not known whether dreaming occurs tonically or phasically during REM sleep.
The synchronizer (pacemaker) for the sleep–wake cycle appears to reside in the suprachiasmatic nucleus of the hypothalamus,27,28 but the generators or executors of the various REM phenomena, both tonic and phasic, are located in the pons.17,29,30
The tonic and phasic neurophysiologic processes underlying each state can be variously dissociated and recombined across states.31 For REM sleep, the processes that generally occur in concert may also be seen in dissociated form, both experimentally (e.g., REM sleep–deprived animals with PGO spikes occurring in NREM sleep and wakefulness)32 and in human and animal disease (narcolepsy). In narcolepsy, the best-understood dissociated state, the sleep attacks, hypnagogic hallucinations, sleep paralysis, cataplexy, and automatic behavior each represent the intrusion or persistence of one state of being into another (i.e., cataplexy may be the inappropriate isolated intrusion of REM sleep atonia into wakefulness, usually induced by an emotionally laden event).33,34 RBD likewise represents a dissociated state: REM sleep containing one element of wakefulness—muscle tone.
Epidemiology and Risk Factors
A recent phone survey of more than 4900 persons between the ages of 15 and 100 years of age indicated an overall prevalence of violent behavior in general during sleep of 2%, one quarter of which were likely due to RBD, giving an overall prevalence of RBD at 0.5%.35 Another survey estimated the prevalence of REM sleep behavior to be 0.38% in elderly persons.36 Increasingly, RBD is becoming associated with underlying neurologic conditions.
RBD and Extrapyramidal Disease
As more patients with “idiopathic” RBD are carefully followed over time, it is becoming clear that the majority eventually develop neurodegenerative disorders, most notably the synucleinopathies: Parkinson’s disease, multiple system atrophy (MSA; including olivopontocerebellar degeneration and the Shy-Drager syndrome), dementia with Lewy body disease, or pure autonomic failure. RBD may be the first manifestation of these conditions, and it can precede any other manifestation of the underlying neurodegenerative process by more than 10 years.37–47
Combined animal and human studies have identified physiologic and anatomic links between RBD and neurodegenerative disorders, leading to the proposal that neurodegeneration can begin in either the rostroventral midbrain or the ventral mesopontine junction and progressively extend to the rostral or caudal part of the brainstem. When the lesion starts in the ventral mesopontine region, RBD develops first, but when the lesion initially involves the rostroventral midbrain, Parkinson’s disease is the initial manifestation.48
Systematic longitudinal study of patients with such neurologic syndromes indicates that RBD and REM sleep without atonia may be far more prevalent than previously suspected. Although the prevalence of RBD in Parkinson’s disease is unknown, subjective reports indicate that 25% of patients with Parkinson’s disease have sleep-related behavior suggesting RBD or they have sleep-related injurious behavior, and polysomnographic studies found RBD in up to 47% of patients with Parkinson’s disease who had sleep complaints.49–52 In one large series of patients with MSA, 90% were found to have REM sleep without atonia and 69% had clinical RBD.53 In another, nearly half had RBD.54 The presence of RBD might differentiate pure autonomic failure from MSA with autonomic failure.55 The finding of incidental Lewy body disease in one patient asymptomatic for Parkinson’s disease suggests that this condition might explain idiopathic RBD in some older patients.56 The presentation of RBD and dementia is suggestive enough of dementia with Lewy body disease that RBD has been proposed as one of the core diagnostic features of dementia with Lewy body disease.57
The link between Parkinson’s disease and RBD (Video 95-1![]() ) is supported by the fact that impaired olfactory and color discrimination is common to both.58–59a Also, the possible presence of cognitive deficits and slowing in the waking electroencephalogram in idiopathic RBD share common features with dementia with Lewy body disease.60–62 RBD is also seen in non–synucleinopathy-related Parkinson’s disease, in Guadeloupian parkinsonism, and progressive supranuclear palsy (tauopathies).63–65 The clinical features of RBD are identical in the idiopathic cases and in those with Parkinson’s disease or MSA.66 Interestingly, there is a striking (77%) male predominance in patients with Parkinson’s disease who display RBD.67 To date, no reliable tests have been shown to identify which subgroup of patients with idiopathic RBD is prone to develop Parkinson’s disease.68
) is supported by the fact that impaired olfactory and color discrimination is common to both.58–59a Also, the possible presence of cognitive deficits and slowing in the waking electroencephalogram in idiopathic RBD share common features with dementia with Lewy body disease.60–62 RBD is also seen in non–synucleinopathy-related Parkinson’s disease, in Guadeloupian parkinsonism, and progressive supranuclear palsy (tauopathies).63–65 The clinical features of RBD are identical in the idiopathic cases and in those with Parkinson’s disease or MSA.66 Interestingly, there is a striking (77%) male predominance in patients with Parkinson’s disease who display RBD.67 To date, no reliable tests have been shown to identify which subgroup of patients with idiopathic RBD is prone to develop Parkinson’s disease.68
The waking motor impairments of Parkinson’s disease can improve or even normalize during REM sleep–related movements in Parkinson’s disease patients with RBD. In a study of 53 patients with Parkinson’s disease and RBD who slept with bed partners, 100% reported improvement of at least one of the following during RBD episodes: faster, stronger, or smoother movements; more intelligible, louder, or better articulated speech; or normalization of facial expression. Furthermore, 38% of bed partners reported that movements were “much better” even in the most disabled Parkinson’s disease patients. The responsible mechanisms for these fascinating observations remain obscure.69
RBD and Narcolepsy
RBD might also be yet another manifestation of narcolepsy. It is present in more than half of patients with narcolepsy, it may be an early symptom in childhood narcolepsy, and it might even be the presenting symptom in narcolepsy.70–74 These patients usually have hypocretin-1 deficiency.72 Tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), and selective serotonin reuptake inhibitors (SSRIs), prescribed to treat cataplexy, can trigger or exacerbate RBD in this population. The demographics (age and sex) of RBD in narcolepsy conform to those of narcolepsy, indicating that RBD in these patients is yet another manifestation of state boundary dyscontrol seen in narcolepsy.75
RBD and Other Conditions
Other conditions reported to be associated with RBD include mitochondrial encephalomyopathy, normal pressure hydrocephalus, Tourette’s syndrome, Machado-Joseph disease (spinocerebellar ataxia type 3), cerebellopontine angle tumors, group A xeroderma, multiple sclerosis, ischemic or hemorrhagic cerebrovascular disease, focal brain lesions, autism, Möbius syndrome, voltage-gated potassium channel autoimmunity, and Guillain-Barré syndrome.76–91
Pathogenesis
The generalized atonia of REM sleep results from active inhibition of motor activity by pontine centers of the peri–locus coeruleus region, which exert an excitatory influence upon the reticularis magnocellularis nucleus of the medulla via the lateral tegmentoreticular tract. The reticularis magnocellularis nucleus, in turn, hyperpolarizes spinal motor neuron postsynaptic membranes via the ventrolateral reticulospinal tract.92,93 Loss of muscle tone during REM sleep is very complex and has been shown to result from a combination of inactivation of brainstem motor inhibitory systems and inactivation of brainstem facilitative systems.94,95 Normally the atonia of REM sleep is briefly interrupted by excitatory inputs that produce the rapid eye movements and the muscle jerks and twitches characteristic of REM sleep.96–98 REM atonia is thought to be mediated by glycine99 and may be influenced by medullary enkephalinergic neurons.100 The prevailing hypothesis that REM atonia is caused by glycinergic inhibition has been questioned.101
Bilateral pontine tegmental lesions in cats result in persistent absence of REM atonia associated with prominent motor activity during REM sleep.3,15,24,93,102–104 That this represents REM sleep rather than waking activity is supported by the presence of other features typical of REM: loss of thermoregulation, the closed nictitating membrane, miotic pupils (despite signs of autonomic activity), and blunted response to stimuli.22
Cats receiving pontine tegmental lesions exhibit a range of REM sleep activities that can appear as soon as the second day after the lesion has been produced. Loss of REM atonia has been shown to be necessary, but not sufficient, to allow the expression of REM activities. The specific site of a pontine lesion determines whether loss of atonia occurs with simple movements or more complex activities suggesting that the pontine tegmentum is responsible for two separate mechanisms of skeletal motor inhibition during REM sleep: the atonia system and a system that suppresses phasic brainstem motor pattern generators.15 A lesion damaging the atonia mechanism would produce only REM sleep with augmented tone (REM without atonia [RWA]), whereas a lesion affecting both mechanisms (tonic and phasic), would also release complex activities, with the stereotypical repertoire depending on the precise location of the lesion.
Although the classic experimental animal model of RWA involves bilateral perilocus coeruleus lesions,3 it is clear that other regions of the central nervous system can affect muscle tone during REM sleep, including the medulla105 and possibly even the hypothalamus.30 Relevant animal studies have indicated a co-localization of the locomotor and atonia systems operating during REM sleep.106 Given the clear neuroanatomic substrate of a REM behavioral syndrome in cats, experiments of nature could be expected, resulting in an analogous disorder in humans. The pathophysiology of RBD in humans may be presumed to be similar to that postulated for experimental cats,15 namely the loss of REM atonia coupled with enhancement of phasic motor drive.
Neuroimaging studies indicate dopaminergic abnormalities in RBD. Single photon emission computed tomography (SPECT) studies have found reduced striatal dopamine transporters,107,108 and decreased striatal dopaminergic innervation has been reported.109 Decreased blood flow in the upper portion of the frontal lobe and pons has been reported,110 as has functional impairment of brainstem neurons.111 Positron-emission tomography (PET) and SPECT studies have revealed decreased nigrostriatal dopaminergic projections in patients with MSA and RBD.112 Decreased blood flow in the upper portion of the frontal lobe and pons has been found in one magnetic resonance imaging (MRI) and SPECT study.110 Impaired cortical activation as determined by electroencephalographic spectral analysis in patients with idiopathic RBD supports the relationship between RBD and neurodegenerative disorders.113
RBD in humans occurs in an acute and a chronic form. Until recently, most reported cases of acute transient RBD fell in the toxic or metabolic category, and the best-studied conditions were the withdrawal states, most commonly involving ethanol.6 Comparable patterns have been described with nitrazepam withdrawal and biperiden intoxication.7,8
Currently, the most common cause of acute RWA and RBD may be iatrogenic. Acute RBD is almost always induced by medications (most commonly tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), or selective serotonin reuptake inhibitors (SSRIs)) or associated with withdrawal (alcohol, barbiturate, or meprobamate).114,115 Excessive caffeine ingestion has also been implicated,116 as has chocolate ingestion.117
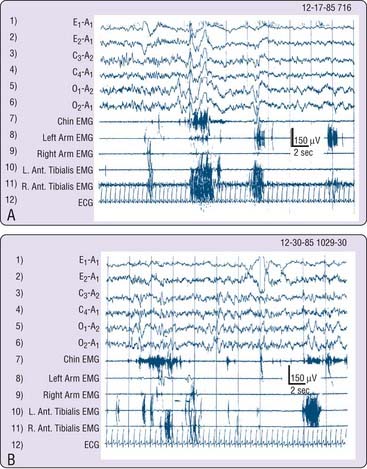
The chronic form is most often either idiopathic or associated with neurologic disorders. Each basic category of neurologic disease (vascular, neoplastic, toxic or metabolic, infectious, degenerative, traumatic, congenital, and idiopathic) could be expected to manifest this disorder. Although RBD has not been reported following infection or trauma, a recent study of persistent hypersomnolence following Epstein-Barr viral infection (infectious mononucleosis and Guillain-Barré syndrome)118 and a case with absent REM sleep as a sequela to a strategically located pontine shrapnel fragment119 indicate that these categories will eventually be implicated. A familial association has been documented120 (Fig. 95-1) and is occasionally suggested historically during clinical evaluations. Spontaneously occurring idiopathic RBD has been reported in dogs and cats.121,122
The overwhelming male predominance of RBD (not seen in the associated neurodegenerative disorders) raises the intriguing question of hormonal influences, as suggested in male-aggression studies in animals and humans.123–125 Another possible explanation for the male predominance is sex differences in brain development and aging.126–128 There is evidence for a sex difference in the effects of sex steroids on the development of the locus coeruleus in rats.129 However, serum sex hormone levels are normal in idiopathic RBD or RBD associated with Parkinson’s disease.130,131
The typically late-age onset of RBD suggests an organic brain factor and may be a manifestation of the reversal or disintegration of ontogeny of state appearance.132 One highly speculative but tantalizing etiologic possibility is that RBD represents a delayed manifestation of REM sleep abnormalities occurring early in development. This explanation invokes the well-documented prolonged effect of pharmacologic manipulation upon developing neural tissues.133 In rats, Corner has described an RBD-like polysomnographic (PSG) pattern persisting into adulthood following clomipramine-induced neonatal REM sleep suppression.134 Another is the possible presence of neuronal-specific antibodies as described in neurologic paraneoplastic syndromes and the stiff-man syndrome.135 One study failed to identify anti–locus coeruleus–specific antibodies in patients with RBD.136 As autopsy material becomes available, direct neuropathologic examination could provide important correlative information. The chronic idiopathic category represents patients whose RBD is not associated with psychopathology or detectable neuropathology.
Clinical Features
The cases reported to date have strikingly similar clinical features.114 The presenting complaint is that of vigorous sleep activities usually accompanying vivid striking dreams (Videos 95-2 and 95-3![]() ). These activities can result in repeated injury, including ecchymoses, lacerations, and fractures. Some of the self-protection measures taken by the patients (tethering themselves to the bed, using sleeping bags or pillow barricades, or sleeping on a mattress in an empty room) reveal the recurrent and serious nature of these episodes.137,138 The potential for injury to the patient or the bed partner raises interesting and difficult forensic medicine issues.139 RBD may have serious psychological ramifications for the bed partner: One woman committed suicide because her husband with RBD could not share their bed.140 Chronic RBD is more common in older men and may be preceded by a lengthy prodrome. In some cases there is a familial predisposition. Because effective and safe treatment is available, precise diagnosis is critical.
). These activities can result in repeated injury, including ecchymoses, lacerations, and fractures. Some of the self-protection measures taken by the patients (tethering themselves to the bed, using sleeping bags or pillow barricades, or sleeping on a mattress in an empty room) reveal the recurrent and serious nature of these episodes.137,138 The potential for injury to the patient or the bed partner raises interesting and difficult forensic medicine issues.139 RBD may have serious psychological ramifications for the bed partner: One woman committed suicide because her husband with RBD could not share their bed.140 Chronic RBD is more common in older men and may be preceded by a lengthy prodrome. In some cases there is a familial predisposition. Because effective and safe treatment is available, precise diagnosis is critical.
Case Studies
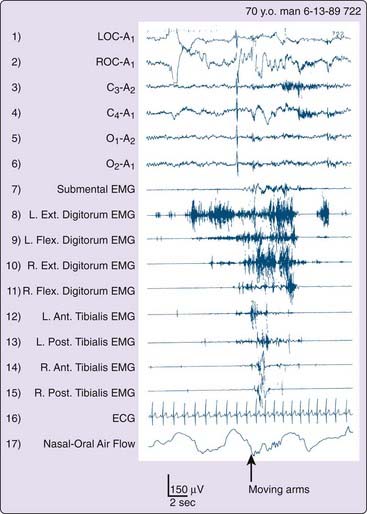
The following cases illustrate the idiopathic subtype of RBD and describe the main reason for seeking medical attention. Past history and current neurologic and psychiatric evaluations were unremarkable, apart from the findings reported. All four men were known by day to be calm and friendly people whose nocturnal behavior was completely out of (waking) character. Clonazepam controlled problematic activities and abnormal dreams in each. Figure 95-2 illustrates attempted dream enactments during the REM sleep of the patient described in Case 3.
Case 4
A 57-year-old retired school principal presented with concern over the possibility of injuring his wife. For 2 years he had inadvertently punched and kicked her during vivid nightmares of protecting himself and family from aggressive people and snakes. Nocturnal arousals were uncommon and he felt refreshed most mornings. He developed an adjustment disorder with depressed mood141 subsequent to a myocardial infarction 1 year previously, but treatment with desipramine and trazodone did not control his problematic sleep movements. The Minnesota Multiphasic Personality Inventory (MMPI) revealed a chronic tendency for somatization. Two brothers reported identical sleep and dream disturbances that had persisted since adolescence. One of them tore the headboard off a bed while dreaming of fighting a bear.
Behavioral Features and their Bases
The history of dream-enacting behavior occurring at least 90 minutes after sleep onset and as late as the terminal morning awakening strongly suggests a REM sleep disorder, and in our laboratory nearly all such episodes take place within REM sleep. One rare example of a NREM dream enactment (see Fig. 1 in reference 10) was actually a dissociated state in which an RBD process intruded into the transition from stage 3-4 to stage 2 sleep with REMs, a vivid dream, and behavioral enactment. This case most likely represents a parasomnia overlap state (discussed later in the section “Variations of REM Sleep Behavior Disorders”).
Two conclusions can be drawn. First, chronic RBD is principally a motor disorder and uncommonly also an arousal disorder. Second, the very high arousal threshold constitutes another physiologic marker of REM sleep, which is known to have the highest threshold for arousal compared to wakefulness or NREM sleep.18–20 In addition, the autonomic nervous system was generally not activated during episodes of vigorous REM sleep activities, as indicated by Figure 95-2. However, a few patients described episodes of vivid dreaming with behavioral enactment from which they awakened in a terrified state with full subjective autonomic activation.
In RBD patients, arousal from sleep to alertness and orientation is usually rapid and accompanied by complete dream recall (very unlike the confusional arousals observed in the disorders of arousal such as sleepwalking or sleep terrors). After awakening, behavior and social interactions are appropriate, mitigating against a NREM sleep relationship, delirious states, or ictal phenomena, and further supporting a REM sleep phenomenon. The activities, although complex and violent, are of briefer duration than those seen in the disorders of arousal. In some patients, the clinical features contain elements of both RBD and disorders of arousal (see later). Furthermore, appetitive behavior (feeding or sexual) has not been seen as a manifestation of RBD in humans or in the animal model.15
Dream Disorder and Behavior Disorder
The Hobson and McCarley activation-synthesis model of dream formation states that during REM sleep, brainstem generators phasically activate motor, perceptual, affective, cognitive, and amnestic circuits whose information flow is synthesized into dreams by the forebrain.142,143 This model would theoretically predict the dream changes observed in patients with RBD. Intensified activity of these generators or biased activation of particular circuits should induce corresponding changes in dream process and content. We theorize that both these conditions occur to produce RBD. As already proposed for cats with pontine lesions, disinhibition of selective brainstem motor pattern generators accounts for the differential release of stereotypical REM behavior.15 This same process might produce both the behavior and dream disorder of RBD. For example, the generator for violent behavior might become disinhibited and coactivate both a descending output to the spinal motor neurons and an ascending output to forebrain dream-synthesizing centers, thus producing the simultaneous movement and dreaming. These two outputs from the same generator may be isomorphic, so that command for dream action (fictive movements) is equivalent to command for actual movements,144 resulting in acting out of dreams.
A singular feature of the dream-enacted episodes in this group of patients is that customary dreams are generally not being played out; rather, distinctly altered, stereotypical, repetitive, and action-packed dreams are put on display. The violence of the sleep-related behavior is often discordant with the waking personality. The increased aggressive dream content experienced by patients with RBD is not associated with increased daytime aggressiveness.145
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree