FIGURE 9-1 The prevalence of four risk factors (obesity, diabetes, hypertension, and cardiovascular disease) in patients screened in the Kidney Early Evaluation Program, grouped by the stage of chronic renal disease. (Reproduced from Whaley-Connell AT, Sowers JR, Stevens LA, et al. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 2008;51:S13–S20.)
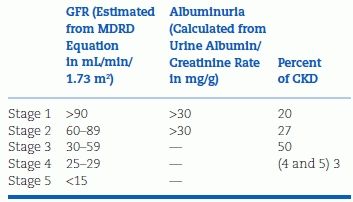
TABLE 9-1 Classification of Chronic Kidney Disease
By 2020, with the aging of the population and the increasing prevalence of diabetes, nearly 150,000 persons in the U.S. are projected to begin therapy for ESRD, nearly 800,000 will be living on chronic dialysis or with a kidney transplant, and costs for ESRD are projected to reach $54 billion.
The Role of Hypertension
Hypertension is second only to diabetes as the primary cause of ESRD. Even prehypertension, defined as blood pressure (BP) >120/80 mm Hg but <140/90 mm Hg, is associated with an increased risk of the onset of CRD (Huang et al., 2014). Moreover, higher nocturnal levels of BP, unrecognized without rarely performed 24-hour ambulatory monitoring, have been found to be much more closely related to the development of CRD than are daytime readings (Kanno et al., 2013). Unfortunately, in blacks with CRD, higher nocturnal BPs persisted despite the dosing of antihypertensive drugs at bedtime (Rahman et al., 2013).
Overall, few CKD patients have their BP adequately controlled to below 140/90 mm Hg: Only 13.2% of over 10,000 people screened in the Kidney Early Evaluation Program were well controlled, although 80% of these participants were aware of their hypertension and 70% were being given antihypertensive medication (Sarafidis et al., 2008a). Those who were poorly controlled more likely had elevated systolic pressure and were more likely elderly, obese, black, and male.
These data are likely related to two factors. First, since RRT for ESRD is totally compensated by Medicare, black men have unrestricted access to RRT (Duru et al., 2008) and actually do better than Caucasian men when they start dialysis (Norris et al., 2008). On the other hand, black men are much less likely to receive medical care that could prevent their progressing into ESRD (Evans et al., 2011) reflecting the absence of a rational health care system in the U.S. Unfortunately, black men in particular, and poor people in general, will continue to suffer the consequences of a skewed health care delivery system willing to pay millions to keep ESRD patients alive but unwilling to pay hundreds to prevent their progression into ESRD. The waste, in both money and suffering inherent in the U.S. system, is seen when our data are compared to countries with universal access to care: Norway has the same prevalence of CKD as the U.S., but the rate of progression from stage 3 to stage 4 and to ESRD is threefold higher in the U.S. (Hallan et al., 2006).
Poverty and limited access to comprehensive health care add yet another mechanism for CKD—inadequate nutrition during pregnancy and prematurity, which diminish renal development in low birth weight babies (Luycks et al, 2013).
Practical Solutions
As societal changes are being sought, two simple changes in current practices need implementation:
First, increase the performance of spot urine testing for albuminuria and calculation of eGFR from a serum creatinine (Rule et al., 2013) or cystatin C (Shlipak et al., 2013). Second, encourage primary caregivers to treat those with stage 1 or 2 disease more intensively. There are not enough nephrologists to care even for those with stage 3 disease, which is the level of CKD that is now the criterion for referral to a nephrologist. Table 9-2 provides a nine-item list for prevention of the progression of kidney damage (Graves, 2008).
TABLE 9-2 Measures to Prevent Progression of Kidney Disease

SI conversion factor: To convert LDL-C values to mmol/L, multiply by 0.0259.
LDL-C, low-density lipoprotein cholesterol; NSAIDs, nonsteroidal anti-inflammatory drug. Modified from Graves JW. Diagnosis and management of chronic kidney disease. Mayo Clin Proc 2008;83:1064–1069.
We will now examine the specific varieties of renal disease and how they relate to hypertension, starting from acute renal insults and progressing eventually to posttransplantation. Renovascular hypertension is covered in the next chapter. It should always be kept in mind as a potentially curable form of CKD.
ACUTE KIDNEY DISEASE
A rapid decline in renal function may appear from various causes: prerenal (e.g., volume depletion), intrinsic (e.g., glomerulonephritis), or postrenal (e.g., obstructive uropathy). Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most common causes of acute renal failure, particularly in patients whose already reduced renal perfusion depends on prostaglandin-mediated vasodilation.
Acute Kidney Injury
Acute kidney injury (AKI) is defined differently in different studies (Zappitelli et al., 2008). Perhaps the best is the RIFLE classification, which provides a gradation of severity, starting with stage 1 or “risk” as oliguria for more than 6 hours or an increase in serum creatinine of more than 50% and proceeding to stage 2 as “injury” and stage 3 as “failure” with greater disease severity (Kellum et al., 2008).
Waikar et al. (2008), in their review of AKI, list these as the most common precipitants, all more common in the elderly, the diabetic, and those with preexisting CKD (Hsu et al., 2008):
- Sepsis
- Coronary interventions: catheterization, angioplasty, and bypass surgery
- Aortic aneurysm repair
- Intravenous contrast for radiologic examinations
- Nephrotoxic antibiotics
Gadolinium and Nephrogenic Systemic Fibrosis
Contrast agents have long been known to reduce renal function (Weisbord et al., 2008), but a more specific syndrome—nephrogenic systemic fibrosis—has been identified as a serious consequence of the use of gadolinium as contrast for magnetic resonance imaging in patients with preexisting ESRD (Kallen et al., 2008).
Recognition of AKI
The need for an early recognition of AKI is obvious, since immediate correction of causative factors is critical for survival. Among many markers that have been proposed, the plasma and urine measurements of neutrophil gelatinase–associated lipocalin (NGAL) are the most promising. NGAL is one of the most rapidly induced proteins in the kidney after acute injury (Mishra et al., 2003), and it can easily be measured in one drop of blood or 0.2 mL of urine (Devarajan, 2008). In a prospective cohort study of 635 patients with suspected AKI, the urinary NGAL provided a 90% sensitivity and a 99.5% specificity, superior to other markers and highly predictive of clinical outcomes (Nickolas et al., 2008).
Hypotension, rather than hypertension, is frequent in many AKI patients because vasodilation and volume depletion may occur at the onset of the injury. If hypertension supervenes, it often reflects iatrogenic volume overload in an attempt to increase renal perfusion. Renin released from hypoperfused kidneys may also be involved.
Acute Glomerulonephritis
The classic presentation of acute glomerulonephritis is a child with recent streptococcal pharyngitis or impetigo who suddenly passes dark urine and develops facial edema. The renal injury represents the trapping of antibody–antigen complexes within the glomerular capillaries. Although the syndrome has become less common, it still occurs, sometimes in adults past middle age. Typically, in the acute phase, patients are hypertensive, and there is a close temporal relation between oliguria, edema, and hypertension. On occasion, hypertension of a severe, even malignant, nature may be the overriding feature.
The hypertension should be treated by salt and water restriction and, in mild cases, diuretics and other oral antihypertensives. In keeping with an apparent role of renin, ACEI and ARB therapies have been effective (Catapano et al., 2008). In the classic disease, the patient is free of edema and hypertension within days, of proteinuria within weeks, and of hematuria within months. Hypertension was found in only 3 of 88 children followed up for 10 to 17 years (Popovic-Rolovic et al., 1991).
More common than poststreptococcal glomerulonephritis are a variety of primary renal diseases (e.g., IgA nephropathy) and systemic diseases (e.g., systemic lupus erythematosus), which may present with acute renal crises marked by hypertension (Haas et al., 2008). The hypertension may be effectively treated with an ACEI or an ARB (Catapano et al., 2008).
Various viral infections may precipitate renal damage, more likely chronic than acute (Berns & Bloom, 2008). HIV-infected patients may present with a continuum from only microalbuminuria (Baekken et al., 2008) to severe antiglomerular basement membrane disease (Wechsler et al., 2008), manifested by heavy proteinuria (Rhee et al., 2008) or malignant hypertension (Morales et al., 2008).
Urinary Tract Obstruction and Reflux
Vesicoureteric reflux is seen in 1% to 2% of otherwise normal children and can lead to hypertension, renal scarring, and ESRD (Gargollo & Diamond, 2007). Hypertension may develop after obstruction of the urinary tract, either unilateral (Shin et al., 2008) or bilateral (Kiryluk et al., 2008). In most patients, the hypertension is fairly mild, but significant hypertension and severe renal insufficiency may occur with hydronephrosis from prostatic obstruction (Sacks et al., 1989). Catheter drainage of the residual urine may lead to rapid resolution of the hypertension and circulatory overload (Ghose & Harindra, 1989).
Other Causes of Acute Renal Disease
Other causes of acute renal disease with hypertension include the following:
- Bilateral renal artery occlusion, by either emboli or thromboses (Svarstad et al., 2005).
- Removal of angiotensin II support of blood flow with ACEI or ARB therapy in the presence of bilateral renal artery disease (Safian & Textor, 2001).
- Trauma to the kidney (Watts & Hoffbrand, 1987).
- Cholesterol emboli, which may shower the kidney after radiologic or surgical procedures, producing rapidly worsening renal function and hypertension (Vidt, 1997).
- Extracorporeal shock wave lithotripsy for kidney stones is only rarely followed by rises in BP (Eassa et al., 2008).
Renal Donors
Removal of half of a living donor’s renal mass could be looked upon as an acute injury, but in normal humans, the removal of a kidney does not usually result in hypertension, likely because of downward adjustments in glomerular hemodynamics to maintain normal fluid volume (Kasiske et al., 2013). In a meta-analysis of 48 studies with 5,145 donors whose average age at donation was 41 years and whose BP averaged 121/77, follow-up for at least 5 years revealed a 6/4–mm Hg increase in BP (Boudville et al., 2006). However, at a mean follow-up of 12.2 years, the survival and incidence of ESRD were similar in 255 donors compared to those in the general population (Ibrahim et al., 2009). No additional risk for cardiovascular events has been seen in kidney donors than in the general population (Garg et al., 2012).
CHRONIC KIDNEY DISEASE
Of the various discernable primary causes of ESRD among patients starting dialysis in the U.S., diabetic nephropathy is the most common, comprising about 40%, followed by vascular diseases, including hypertensive nephrosclerosis (20%), primary glomerular disease (18%), tubulointerstitial diseases (7%), and cystic diseases (5%) (Whaley-Connell et al., 2008).
There are some differences in the prevalence of hypertension and the responses to antihypertensive therapy among these various causes of kidney disease: Chronic pyelonephritis may be less commonly associated with hypertension (Goodship et al., 2000); polycystic diseases may be more commonly associated (Grantham, 2008), even before significant renal dysfunction develops (Reed et al., 2008). Patients with these various causes of CKD may start at either end of the spectrum: Hypertension without overt renal damage on the one end and severe renal insufficiency without hypertension on the other. Eventually, however, both groups move toward the middle—renal insufficiency with hypertension—so that hypertension is found in approximately 85% of patients with CKD of diverse causes (Sarafidis et al., 2008a) and is closely related to the progression of nephropathy. Renal insufficiency as a consequence of primary hypertension is described in Chapter 4. Attention will be directed to data associating genetic variants in blacks rather than “hypertensive nephrosclerosis” as the mechanism for their higher prevalence of CRD, in particular focal segmental glomerulosclerosis (FSGS) (Parsa et al., 2013).
This section examines the development of hypertension as a secondary process in the presence of primary renal disease or diabetes. The special features of diabetic nephropathy are covered separately, but most cases of CKD are similar in their course and treatment. Moreover, almost half of patients clinically defined as having diabetic nephropathy have been shown to actually have nondiabetic renal disease by kidney biopsy (Zhou et al., 2008).
Patients whose underlying problem is bilateral renovascular disease may present with refractory hypertension and renal insufficiency (Guo et al., 2007). The recognition of the renovascular etiology of these patients’ condition is critical because revascularization may relieve their hypertension and improve their renal function. More about this important group of patients with ischemic nephropathy is provided in the next chapter, as well as hypertension associated with renal tumors.
The Role of Hypertension
Hypertension accelerates the progression of renal damage, regardless of the cause. Perhaps the best evidence for this tight relationship is the repeatedly observed slowing of the progression of established CKD as initially elevated BPs are lowered. This was demonstrated first for patients with diabetic nephropathy (Mogensen, 1976) and subsequently for those with other causes of CKD, as in the Modification of Diet in Renal Disease (MDRD) Study (Lazarus et al., 1997). In the MDRD trial, 585 patients with a glomerular filtration rate (GFR) between 25 and 55 mL/minute and 255 patients with a GFR between 13 and 24 mL/minute were studied. Among those with proteinuria of more than 1 g/day at baseline, the rate of decline in GFR was significantly less over a mean follow-up of 2.2 years in both groups whose BPs remained an average of 5 mm Hg lower as a result of more intensive therapy.
Along with their higher prevalence of hypertension, African Americans have an increased susceptibility to CKD and ESRD. Nondiabetic CKD in African Americans has been attributed to “hypertensive nephrosclerosis,” i.e., hypertension-causing CKD. The diagnosis is usually made by exclusion and with nonspecific FSGS on biopsy. However, missense mutations in the APOL1 gene (initially attributed to the nearby MYH9 gene (Kao et al., 2008; Kopp et al., 2008)) have explained the increased prevalence of CRD in blacks (Tzur et al., 2010).
In patients with CKD, ambulatory BP monitoring, which often identifies a loss of nocturnal dipping, is better than office readings in predicting progression of renal damage and mortality (Pogue et al., 2009). Nondipping in CKD has been attributed to a compensation for diminished natriuresis during the daytime and to enhanced pressure–natriuresis during the night (Kimura, 2008). Out-of-office BP measurements in patients with CKD are also critical to identify the considerable proportion with white-coat hypertension, overtreatment (De Nicola et al, 2013), to avoid unnecessary and potentially harmful overtreatment.
Mechanisms
Hypertension develops and progresses in patients with renal diseases for multiple reasons (Table 9-3). Most of these funnel into a common path: Impaired renal autoregulation that normally attenuates the transmission of elevated systemic pressure to the glomeruli, resulting in high perfusion pressure (Mori et al., 2008). The resultant glomerular hypertension damages the glomerular cells and leads to progressive sclerosis, setting off a vicious cycle (Anderson & Brenner, 1989) (Fig. 9-2).
TABLE 9-3 Features Associated with High BP in Chronic Kidney Disease

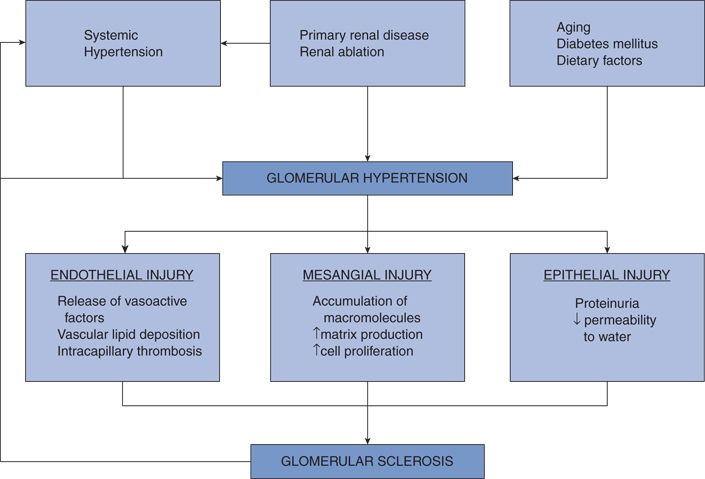
FIGURE 9-2 Pivotal role in glomerular hypertension in the initiation and progression of structural injury. (Modified from Anderson S, Brenner BM. Progressive renal disease: A disorder of adaptation. QJM 1989;70:185–189.)
As the extent of renal damage increases, arteries within the kidneys and throughout the body become sclerotic and stiff. As a consequence, systolic pressure rises, diastolic falls, and pulse pressure widens (Cheng et al., 2008). The stiffness that is responsible for the rising systolic pressure makes it increasingly difficult to lower this pressure. As more and more antihypertensive drugs are added, the systolic barely moves, but the diastolic falls, exposing the CKD patient to potential harm from too low a diastolic pressure to maintain perfusion to the brain, heart, and kidneys (Kovesdy et al., 2013).
Of the contributing or aggravating factors listed in Table 9-3, volume expansion from impaired natriuresis has traditionally been given primacy. However, in view of the increased peripheral vascular resistance typically seen in these patients, both an activated renin–angiotensin–aldosterone mechanism (Hollenberg et al., 2004) and an overactive sympathetic nervous system (Phillips, 2005) have received increasing attention. Obesity, particularly abdominal, accelerates the progression of CKD and the attendant hypertension (Ritz, 2008; Wang et al., 2008).
Proteinuria
The degree of proteinuria serves as a strong predictor of the rate of progression of CKD. Increased protein trafficking through the glomerular capillaries directly damages the podocytes and tubular interstitium (Schieppati & Remuzzi, 2003). The role of heavy proteinuria in progression of renal damage was documented in a meta-analysis of data from 11 randomized controlled trials involving 1,860 patients (Jafar et al., 2003). As seen in Figure 9-3, proteinuria above 1 g/day was associated with a higher relative risk for progression at all levels of systolic BP above 120 mm Hg. The greater the proteinuria, the more the progression. Beyond its inherent toxicity, proteinuria is a useful marker of the type and extent of CKD.
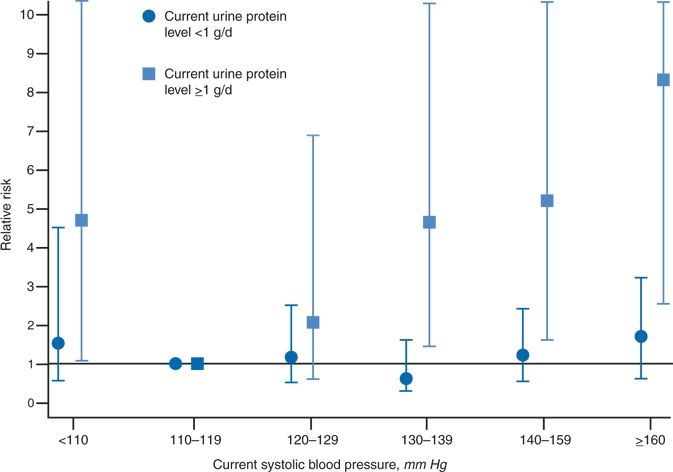
FIGURE 9-3 The relative risk for progression of renal disease in patients with proteinuria either below or above 1 g/day by levels of systolic BP. The reference group (RR = 1) is a systolic pressure of 110 to 119 mm Hg. (Modified from Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease; The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med 2003;139:244–252.)
Measures of Glomerular Filtration Rate
In addition to proteinuria, the presence and degree of CKD are based on the rate of glomerular filtration (see Table 9-1). Until recently, the GFR has been estimated from equations measuring creatinine clearance. These equations have been shown to be less accurate when the GFR is above 60 mL/minute/1.73 m2 and to underestimate measured decreases in renal function over time (Xie et al., 2008). Therefore, attention has turned to the measurement of serum cystatin C, an endogenous protein filtered by glomeruli and reabsorbed and catabolized by tubular epithelial cells with only small amounts excreted in the urine. Unlike equations using serum creatinine, cystatin C levels are not affected by muscle mass, and they are closely correlated to outcomes in patients with CKD (Shlipak et al., 2013).
Management
Intensity of Therapy
Reduction of BP and proteinuria has been clearly shown to slow the rate of progression of CKD (Jafar et al., 2003; Lewis, 2007). However, as seen in Figure 9-3, only those with proteinuria above 1 g/day have been shown to benefit from more intensive lowering of BP. This was found in the MDRD study (Lazarus et al., 1997) and reconfirmed in the African American Kidney Disease and Hypertension (AASK) study (Wright et al., 2002). In the AASK study, no additional slowing of the progression of hypertensive nephrosclerosis was found in those given more therapy to provide an average BP of 128/78 compared to those given less therapy who ended up with an average BP of 141/85. Moreover, 759 of the original 1,094 enrollees in the AASK were followed for another 9 to 12 years while receiving an ACEI (Appel et al., 2010). Despite achieving a mean BP of 133/78, most of the patients continued to suffer a decline in renal function although the decline was less in those with heavy proteinuria.
The reason why there is no benefit of lowered BP on renal function in those with lesser degrees of proteinuria remains unknown. The reason why those with heavy proteinuria benefit likely reflects the damage induced by heavy loads of protein traversing the nephron, and the slowing of this damage as proteinuria is reduced, either by any drug that lowers renal perfusion pressure or by drugs that have a special ability to lower intraglomerular pressure, i.e., renin–angiotensin blockers.
Hazards of More Intensive Therapy
In multiple trials of patients with CKD, an increased incidence of cardiovascular morbidity and mortality has been noted when systolic pressures are lowered to below 130 mm Hg or diastolic pressure below 70 mm Hg (Kovesdy et al., 2013). However, in the Perindopril Protection Against Recurrent Stroke Study (PROGRESS Collaborative Group, 2001), in which all 6,105 participants had known cerebrovascular disease, half were treated with an ACEI plus a diuretic, if needed, to lower the BP. The 1,757 patients with CKD had a progressively lower rate of recurrent stroke with reductions of BP even to below 120/70 mm Hg (Ninomiya et al., 2008).
Lifestyle Changes
All hypertensives with or without CKD, with or without diabetes, should be intensively encouraged to change their unhealthy lifestyles and given as much help as possible to achieve these changes.
Cessation of smoking is paramount, since smoking is a major risk for progression of CKD (Orth & Hallan, 2008).
Reduction in dietary sodium becomes increasingly more critical as CKD progresses and renal sodium excretory capacity becomes weaker (Mimran & du Cailar, 2008). Sodium reduction to the range of 1 to 2 g/day (sodium, 44 to 88 mEq/day) is both feasible and often necessary to control the hypertension in CKD patients (De Nicola et al., 2004). The importance of dietary sodium restriction in proteinuric patients goes beyond the ability to enhance the effect of antihypertensive drugs (Slagman et al., 2011). In a study of 38 patients with CKD and an average of 3.8 g/day proteinuria, reduction of dietary sodium from 196 to 92 mmol/day provided a 22% reduction in proteinuria (Vogt et al., 2008).
Weight reduction: Obese hypertensive people are now likely to develop CKD (Gomez et al., 2006). Abdominal obesity rather than weight per se is the culprit (Elsayed et al., 2008). An increased likelihood of sleep apnea adds to the risk of obesity (Tsioufis et al., 2008).
Renin–Angiotensin System Blockers
Both ACEIs and ARBs reduce proteinuria and slow the progression of CKD equally (Kunz et al., 2008; Sarafidis et al., 2008b). The renoprotective effect has been shown in CKD caused by diabetes (Sarafidis et al., 2008b), nondiabetic disease (Jafar et al., 2003), and polycystic disease (Jafar et al., 2003). Among 28,487 hypertensive adults with a serum creatinine >6 mg/dL, those given an ACEI or ARB had a statistically significant 6% lower risk for requiring long-term dialysis than did those not given a renin–angiotensin system (RAS)-inhibiting drug (Hsu et al., 2013). The prevention of progression to ESRD is directly related to the existing degree of renal impairment (O’Hare et al, 2014).
Despite their benefits, neither ACEIs nor ARBs have been found to reduce all-cause mortality in patients with CKD. Presumably, nonrenal events, which may become increasingly common the longer the CKD is held in check, are responsible.
These better effects of RAS inhibitors likely reflect their greater ability to reduce intraglomerular pressure by their preferential dilation of efferent arterioles (Fig. 9-4) (Tolins & Raij, 1991). The reduction in intraglomerular pressure protects the glomeruli from progressive sclerosis and reduces the escape of protein into the tubule. At the same time, GFR is reduced and serum creatinine increased, usually to only a small degree. This expected, initial slight lowering of GFR is not a cause for stopping the use of an ACEI or ARB and is, in fact, followed by even greater renal protection (Holtkamp et al., 2011). If the serum creatinine rises or GFR falls more than 30% of the pre-ACEI or pre-ARB level, the ACEI or ARB should be stopped and other possible contributing causes identified and corrected, including volume contraction, concomitant use of NSAIDs, or, most dramatically, the presence of bilateral renovascular hypertension.
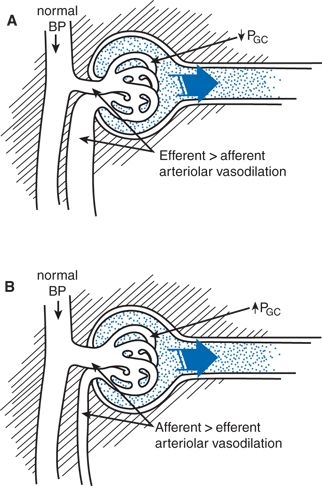
FIGURE 9-4 Effect of antihypertensive treatment on glomerular hemodynamics as determined by micropuncture studies in rats. A: Angiotensin-converting enzyme inhibition results in normalization of BP associated with vasodilation, predominantly of the efferent arteriole, resulting in the normalization of intraglomerular capillary pressure (PGC). B: With CCBs, reduction of BP is offset by afferent arteriolar vasodilation, and, therefore, PGC remains elevated. (Modified from Tolins JP, Raij L. Antihypertensive therapy and the progression of chronic renal disease: Are there renoprotective drugs? Semin Nephrol 1991;11:538–548.)
Another reflection of RAS inhibition is a rise in serum potassium, usually less than 0.5 mEq/L. However, if hyperkalemia above 5.5 mEq/L develops, the dose of ACEI or ARB should be reduced or the drug discontinued. Obviously, blood chemistries should be monitored within a few days of starting ACEI or ARB therapy in patients with CKD as a rapid and sustained rise in serum creatinine may occur with unrecognized bilateral renovascular disease or significant hyperkalemia may develop.
Combination of ACEI and ARB
Kunz et al. (2008) showed that the combination of an ACEI and an ARB reduced proteinuria an additional 20% over that seen with either drug alone. However, in the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET), the combination of the ACEI and the ARB in the same doses as were used alone did not reduce proteinuria more than either drug alone but worsened major renal outcomes (Mann et al., 2008). The worsening was reflected in more hypotension, more doubling of serum creatinine, and more entering dialysis. Messerli (2009) concluded that “dual RAS blockade should no longer be used in clinical practice” as more recently confirmed in a study with an ACEI and an ARB in patients with diabetic nephropathy (Fried et al., 2013).
Anemia with RAS Inhibitors
Both ACEIs and ARBs have been found to reduce hemoglobin levels in CKD patients, an effect attributed to the blockade of erythropoietic effects of angiotensin II on RBC precursors and to the improved oxygenation from increased renal blood flow (Mohanram et al., 2008). In the patients enrolled in the RENAAL trial given the ARB losartan, the greatest effect on hemoglobin was seen at 1 year, but there was no impact on the renoprotective effect of the ARB.
Diuretics
A diuretic will likely be needed to bring the hypertension to near the 130/80–mm Hg goal that current guidelines recommend for CKD patients (Agarwal & Sinha, 2012). A therapeutic cross fire is often encountered: On the one hand, the need for a diuretic becomes progressively greater as renal function deteriorates and sodium cannot be excreted, so the intravascular volume is expanded and the BP rises (Sica, 2008). On the other hand, as renal function deteriorates, diuretics may not work. All diuretics must gain entry to the tubular fluid and have access to the luminal side of the nephron to work. They reach the tubular fluid by secretion across the proximal tubule by way of organic acid secretory pathways. Patients with CKD are thus resistant to acidic diuretics such as thiazides and the loop diuretics because of the accumulation of organic acid end products of metabolism that compete for the secretory pump.
In practice, thiazide diuretics in usual doses (12.5 to 50 mg) are usually not adequate when eGFR falls to below 50 mL/minute/1.73 m2. Fortunately, loop diuretics can be safely given at high enough doses to cross the secretory barrier and exert a diuresis, even with much lower eGFR. To do so, enough must be given by the process of “sequential doubling of single doses until a ceiling dose is reached” (Brater, 1988). Once the ceiling dose is reached, that dose should be given as often as needed as a maintenance dose. If volume control is still not achieved, metolazone alone, or, even better, with a loop diuretic, will usually achieve a diuresis even in ESRD, if some residual renal function is present (Sica & Gehr, 2003). Caution is needed not to overdiurese by carefully monitoring the body weight.
Aldosterone Blockers
Aldosterone is now recognized to be an accelerator of renal damage by stimulating inflammation and fibrosis (Remuzzi et al., 2008). Since its secretion is largely controlled by angiotensin, the suppression of aldosterone synthesis by RAS inhibitors is considered to be responsible for at least part of the overall benefits of RAS inhibitors. However, a breakthrough of aldosterone secretion in the face of continued RAS inhibition has been recognized, first in the treatment of heart failure (Lee et al., 1999), then in the treatment of hypertension (Sato & Saruta, 2001), and then in patients with CKD (Sato et al., 2003). Bomback and Klemmer (2007) identified eight well-performed studies with a range of incidence of breakthrough varying from 10% over 6 months to 53% over 1 year.
When an aldosterone blocker is added to an ACEI or ARB in CKD patients, proteinuria decreases from the level achieved by the RAS inhibitor by 15% to 54% and a significant fall in BP occurs in 40% of the patients (Bomback et al., 2008). Whether these impressive benefits occur only, or usually, in the presence of aldosterone breakthrough is not known, but aldosterone blockers are being increasingly used in CKD patients not adequately managed by RAS inhibition. An aldosterone blocker is usually relegated to the fourth line of therapy and only in those with a normal serum potassium because of the potential for hyperkalemia from the inhibition of potassium excretion by the aldosterone blocker. However, with the recognition of their remarkable ability to bring resistant hypertension into control even in hemodialysis patients (Flevari et al., 2013), the cautious use of aldosterone blockers even earlier in the treatment of CKD may become more acceptable.
Calcium Channel Blockers
As either second or third choice in treatment of hypertension in CRD patients, nondihydropyridine calcium channel blockers (non-DHP-CCBs) have usually been recommended based on their greater antiproteinuric effect than seen with DHP-CCBs in a review of 28 randomized trials (Bakris et al., 2004). This difference is attributed by Bakris et al. to a greater effect of non-DHP-CCBs on efferent arteriolar vasodilation than seen with DHP-CCBs in experimental models (Griffin et al., 1999). In addition, non-DHP-CCBs have been found to reduce glomerular permeability (Russo et al., 2002).
These differences in antiproteinuric effects have not been shown to eventuate in differences in renal protection between DHP-CCBs and non-DHP-CCBs. However, additional concern arose from the AASK trial of patients with CKD from hypertensive nephrosclerosis, wherein those with proteinuria greater than 300 mg/day had a faster decline in GFR if started on the DHP-CCB amlodipine than if started on the ACEI ramipril (Agodoa et al., 2001). However, the majority of the patients in the AASK trial had proteinuria less than 300 mg/day, and among them, GFR was better preserved in those on amlodipine. Moreover, in the Ramipril Efficacy in Nephropathy trial, the use of DHP-CCBs improved renoprotection when added to an ACEI and when BP was reduced effectively (Ruggenenti et al., 1998).
More recently, the combination of a DHP-CCB, either amlodipine or azelnidipine, with the ARB olmesartan lowered BP more and reduced cardiovascular events more than seen by doubling the dose of the ARB (Kim-Mitsuyama et al., 2013). Therefore, either type of CCB can safely and effectively be used when added to an ACEI or ARB in patients with CKD.
α-Blockers
Peripheral α-blockers, e.g., doxazosin, may be used without dose adjustment. The central α-blocker clonidine is often used as a bridge to lower BP on the days between dialysis, but its side effects and propensity to rebound makes it a poor substitute for adequate control of fluid volume.
β-Blockers
Now that their use has been shown to be less effective for primary prevention (see Chapter 7), β-blockers should be used only for secondary prevention of cardiac problems, e.g., post-MI, CHF, or tachyarrhythmias. If one is to be used, the choice should logically be one that is not cleared through the kidney, e.g., propanolol or timolol. The vasodilating α-/β-blocking agents carvedilol and labetalol will cause less metabolic mischief than a β-blocker, and carvedilol has been shown to reduce proteinuria in CKD patients (Bakris et al., 2006). The vasodilating β-blocker nebivolol likely would do as well.
Minoxidil
In the past, those with refractory hypertension and CKD were often treated with minoxidil (Toto et al., 1995). However, when added to a regimen that included maximal doses of an ACEI or ARB, proteinuria increased despite the lower BP (Diskin et al., 2006).
Timing of Therapy
The potential for additional adverse effects of the persistently elevated nocturnal BP, i.e., nondipping, that is frequently present in patients with CKD has prompted studies comparing a shift in the timing of antihypertensive drug intake from morning to evening. Hermida et al. (2005) found a decrease in the level of microalbuminuria among 200 hypertensives when the ARB valsartan was given at bedtime, compared to when it was given in the morning. A similar benefit was reported among 32 CKD patients whose proteinuria was decreased from 235 to 167 mg/day, when they took any one of their average daily intake of 2.4 medications in the evening (Minutolo et al., 2007).
However, as previously noted, bedtime dosing did not reduce the elevated nocturnal BP among black patients in the AASK follow-up study (Rahman et al., 2013).
The addition of a diuretic (Uzu et al., 2005) or the use of a long-acting antihypertensive taken in the morning may reduce the nocturnal pressure, at least in one study by increasing the daytime natriuresis, so the residual vascular volume is reduced (Fukuda et al., 2008). Similar benefit should be provided by a lower dietary sodium intake since, by the nature of CKD, renal sodium excretion is impaired (Bankir et al., 2008).
Restriction of Dietary Protein
A protein-restricted diet has been recommended for predialysis patients (Walser et al., 1999), and an analysis of multiple randomized trials has shown a delay in ESRD or death (Fougue et al., 2000), but individualized decisions seem appropriate in view of the malnutrition often seen with CKD (Levey, 2002).
Correction of Anemia
Anemia is a risk factor for progression of CKD and left ventricular hypertrophy (Rossing et al., 2004). However, treatment with erythropoietin to achieve a hemoglobin level above 12 g/L has been found to increase serious adverse events, so the current recommendations are to maintain a level of 11 g/L (Moist et al., 2008).
Lipid-Lowering Agents
In view of the common presence of dyslipidemia in CKD patients and the high rate of atherosclerotic vascular disease they suffer, the use of lipid-lowering agents seems appropriate. In a review of 50 trials involving 30,144 patients with CKD, statin therapy was found to reduce the risk of cardiovascular morbidity and mortality but had no effect on all-cause mortality and had uncertain renoprotective effects (Strippoli et al., 2008).
Increased Nitric Oxide
The potential of phosphodiesterase type 5 inhibition, now used to treat erectile dysfunction, to increase nitric oxide–induced vasodilation has been proposed (Brown et al., 2014).
Dose Modification of Other Drugs
The presence of CKD can influence the dosing of a variety of drugs, in particular those with considerable renal clearance (Table 9-4) (Kappel & Calissi, 2002). In stage 4 and 5 CKD, the metabolism and transport of nonrenally cleared drugs may also be altered (Nolin et al., 2008).
TABLE 9-4 Dose Modification for Patients with Renal Insufficiency
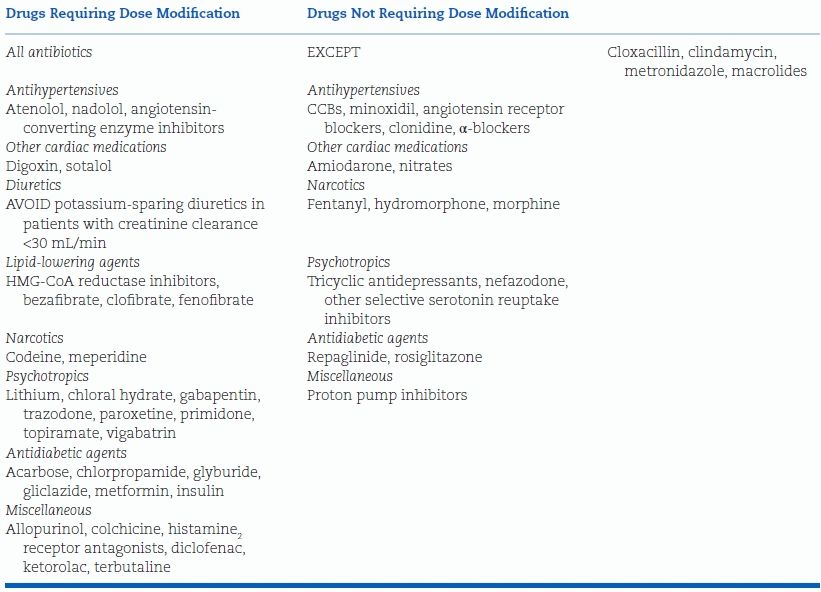
Modified from Kappel J, Calissi P. Nephrology: 3. Safe drug prescribing for patients with renal insufficiency. CMAJ 2002;166:473–477.
Beyond the impact of CKD on the handling of various drugs, used either for its own treatment or for the treatment of concomitant diseases, it is important to recognize the potential for renal damage from both commonly used drugs, e.g., analgesics (Chang et al., 2008), and over-the-counter herbal remedies (Laliberte et al., 2007), as well as newer chemotherapeutic agents (Jain & Townsend, 2007).
CKD in the Elderly
With ageing, renal size and function decrease. Whether these changes are indicative of disease or an expected consequence of ageing is arguable. The presence of hypertension, whether cause or effect, hastens the loss of renal function (Rifkin et al., 2013).
Those over age 65 are becoming the largest burden of CKD: The medium age of new dialysis patients in the U.S. is now 65 years of age, and the fastest growing group of new dialysis patients is of those older than 75 years of age (Stevens et al., 2008) (Fig. 9-5). Although the diminution of renal structure and function with age may largely reflect the impact of nonrenal diseases, e.g., hypertension or diabetes (Rifkin et al., 2013), the kidney ages even in their absence (Rule et al., 2011).
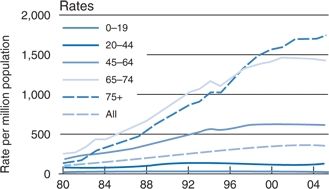
FIGURE 9-5 Rates of new cases of ESRD in patients at various ages treated by dialysis or transplantation in the U.S., by years from 1980 to 2004. (Modified from U.S. Renal data systems. Chronic Kidney Disease. USRDS 2007, Bethesda NIH, NIDDKD 2008.)
The loss of renal function with age is often heralded by increasing nocturia, as sodium ingested during the day is more slowly excreted into the night (Kujubu & Aboseif, 2008). More seriously, cognitive impairment closely accompanies progressive CKD (Barzilay et al., 2011).
More in the U.S. than elsewhere, older patients with advanced CKD are increasingly started on RRT, including dialysis and transplantation. However, the societal costs and the individual discomforts of such intensive treatment are well recognized. Calls for more limited management are being made, particularly for those afflicted with other life-threatening conditions (Schell & Holley, 2013).
DIABETIC NEPHROPATHY
Most of the preceding coverage of CKD applies to the most common of its causes—diabetic nephropathy. However, diabetes provokes additional pathology and requires additional therapy (Table 9-5) (KDIGO, 2012).
TABLE 9-5 Goals for Risk Factor Management in Diabetic Patients

LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HbA1c, hemoglobin A1c.
Pathology and Clinical Features
As delineated by Kimmelstiel and Wilson (1936), renal disease occurs among diabetics with a high incidence and with a particular glomerular pathology—nodular intercapillary glomerulosclerosis. The clinical description has been improved very little since their original paper (Kimmelstiel & Wilson, 1936):
The clinical picture appears… to be almost as characteristic as the histological one: the patients are relatively old; hypertension is present, usually of the benign type, and the kidneys frequently show signs of decompensation; there is a history of diabetes, usually of long standing; the presenting symptoms may be those of edema of the nephrotic type, renal decompensation or heart failure; the urine contains large amounts of albumin and there is usually impairment of concentrating power with or without nitrogen retention.
The clinical description should be altered to include younger patients who have been diabetic for more than 15 years, to involve hypertension in approximately 50% to 60% of patients, and to almost always be accompanied by retinal capillary microaneurysms.
Course
Persistent microalbuminuria, as the first manifestation of diabetic nephropathy, has been observed in about one-third of newly diagnosed type 1 diabetics within 20 years (Hovind et al., 2004) and in about one-quarter of newly diagnosed type 2 diabetics within 10 years (Adler et al., 2003). The difference in time of onset may largely reflect the long asymptomatic background of type 2 compared to the usual abrupt onset of type 1. Rather surprisingly, regression of microalbuminuria has been observed in a significant percentage of type 1 diabetics, generally associated with lower levels of BP and glycemia (Hovind et al., 2004; Perkins et al., 2003).
Nelson et al. (1996) studied renal function every 6 to 12 months over 4 years in 194 Pima Indians who were selected as the representatives of different stages in the development of diabetic nephropathy: from normal glucose tolerance to overt diabetes and from normal albumin excretion to macroalbuminuria. As shown in Figure 9-6, the major findings generally were as follows: Glomerular hyperfiltration is present from the onset until macroalbuminuria appears. Thereafter, GFR declines rapidly because of a progressive loss of intrinsic ultrafiltration capacity. Although the rather abrupt fall in GFR that occurs after approximately 15 years was not prevented by the control of BP, higher baseline pressures predicted an increasing urinary albumin excretion, which in turn mediated a fall in GFR.
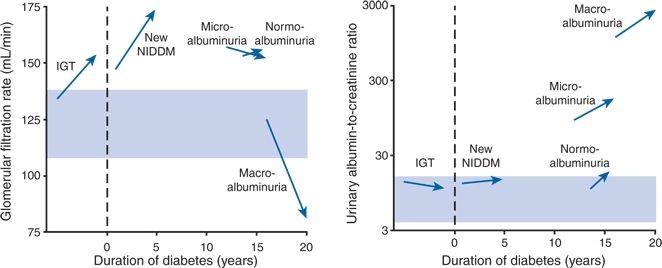
FIGURE 9-6 Changes in the mean GFR and median urinary albumin (mg/L) to creatinine (g/L) ratio from baseline to the end of follow-up in subjects with impaired glucose tolerance (IGT), newly diagnosed non–insulin-dependent diabetes mellitus (New NIDDM), NIDDM and normal urinary albumin excretion (normoalbuminuria), NIDDM and microalbuminuria, and NIDDM and macroalbuminuria. Arrows connect the value of the baseline examination with the value at the end of follow-up; dashed lines indicate the time of diagnosis; shaded areas indicate the 25th and 75th percentiles of values in subjects with normal glucose tolerance. (Modified from Nelson RG, Bennet PH, Beck GH, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 1996;334:1636–1642.)
Mechanisms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








