Data from Eyler WR, Clark MD, Garman JE, et al. Angiography of the renal areas Including a comparative study of renal arterial stenosis In patients with and without hypertension. Radiology 1962;78:879–892.
More recent studies show similar data. ARAS is detected in up to 30% of patients undergoing screening (“drive by”) renal angiography during diagnostic cardiac catheterization (Sattur et al., 2013).
As a historical note, before 1960, unilateral nephrectomy was frequently performed on hypertensive patients with a unilateral small kidney who did not have reversible atherosclerotic renovascular hypertension (AVRHT). Smith (1956) recognized this as early as 1948 as a misguided application of Goldblatt’s experimental model of hypertension induced by clamping the renal artery. Smith reported that only 25% of patients were relieved of their hypertension by nephrectomy and warned that only about 2% of all hypertensives probably could be helped by this surgery.
PREVALENCE OF RVHT
Smith’s (1956) estimate of the true prevalence of ARVHT may be right. The prevalence varies with the nature of the hypertensive population:
- In nonreferred hypertensive patient populations, the prevalence is likely less than 1% (Kalra et al., 2005).
- In patients with resistant hypertension, the prevalence is higher: For example, in one recent series, 24% of 285 patients of mean age 73 with suggestive clinical features had at least a 50% stenosis of one or both renal arteries by renal angiography (Benjamin et al., 2014). The prevalence estimate clearly would be less if a 70% stenosis were required.
- Since most renovascular disease is atherosclerotic in origin, the prevalence, not surprisingly, increases with age and often coexists with peripheral artery disease (Benjamin et al., 2014). ARAS occurs in over 40% of patients with peripheral artery disease and 7% to 14% of patients undergoing diagnostic cardiac catheterization for suspected coronary disease (Boateng & Greco, 2013). Of 1,734 patients (mean age 72) undergoing nonemergent diagnostic coronary catheterization for chest pain, 72% had CAD and 7% had renal artery stenosis by Doppler ultrasound (Imori et al., 2014).
- Diabetic hypertensives, surprisingly, do not have an increased risk of ARAS (Benjamin et al., 2014).
- ARAS may or may not be less common in black hypertensives; data are scarce and ascertainment bias is an issue (Svetkey et al., 1991).
- ARVHT has been recognized in neonates (Ramaswamy et al., 2011) and children (Zhu et al., 2014).
A systemic review of 40 studies involving a total number of 15,879 patients found the following prevalence estimates for ARAS (de Mast & Beutler, 2009):
- Hypertension and diabetes, 20%
- Coronary angiography, 10.5%
- Coronary angiography in hypertensive patients, 18%
- Coronary angiography and suspected renovascular disease, 17%
- Heart failure, 54%
- Peripheral vascular disease, 25%
- Abdominal aortic aneurysm, 31%
- End-stage renal disease, 41%
MECHANISMS OF HYPERTENSION
Animal Models
The pathophysiology of RVHT was first identified by Goldblatt et al. (1934) who—looking not for RVHT but for a renal cause for primary hypertension—put clamps on both main renal arteries of dogs. The clamps were inserted on separate occasions so that they could observe the effect of unilateral obstruction (Fig. 10-1). However, with the modest degree of constriction that they used, unilateral clamping caused only transient hypertension. For permanent hypertension, both renal arteries had to be clamped, or one clamped and the contralateral kidney removed (Goldblatt, 1975).
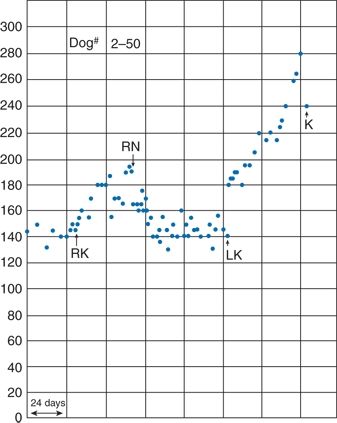
FIGURE 10-1 Results from one of Goldblatt’s original experiments. The graph shows the mean BP of a dog whose right kidney was first moderately constricted (RK), with subsequent hypertension that was relieved after right nephrectomy (RN). After severe constriction of the left renal artery (LK), more severe hypertension occurred, and the animal was sacrificed (K). (From Hoobler SW. History of experimental RVHT. In: Stanley JC, Ernst CB, Fry WJ, eds. Renovascular Hypertension. Philadelphia, PA: Saunders; 1984:12–19.)
After significant renal ischemia and the initial marked rise in renin secretion, renin levels fall but remain inappropriately high and are largely responsible for the hemodynamic changes (Welch, 2000). Figure 10-2 shows a stepwise scheme for the hemodynamic and hormonal changes that underlie ARVHT.
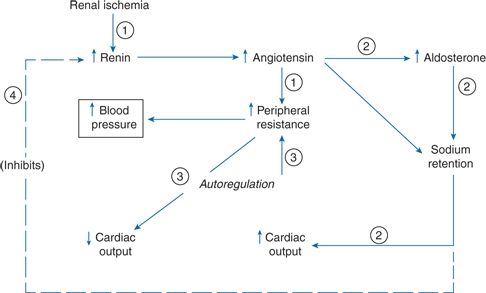
FIGURE 10-2 Hypertension with renovascular disease. Stepwise hemodynamic changes in the development of RVHT.
Activation of the sympathetic nervous system amplifies the effects of renin–angiotensin system activation in the 2-kidney-1-clip animal model of ARAS (Pradhan & Rossi, 2013), a topic pioneered by Page (1982). Renal ischemia activates renal afferent nerves that project to the subfornical organ (SFO), a circumventricular organ that lacks a blood–brain barrier and is thus permeant to circulating angiotensin II (Ang II), which also is increased secondary to increased renin during renal ischemia. These converging neural and hormonal inputs into the SFO activate cardiovascular circuits in the rostral brain stem that increase efferent renal sympathetic nerve activity to the contralateral kidney, further increasing plasma renin activity (Fig. 10-3) (Pradhan & Rossi, 2013).
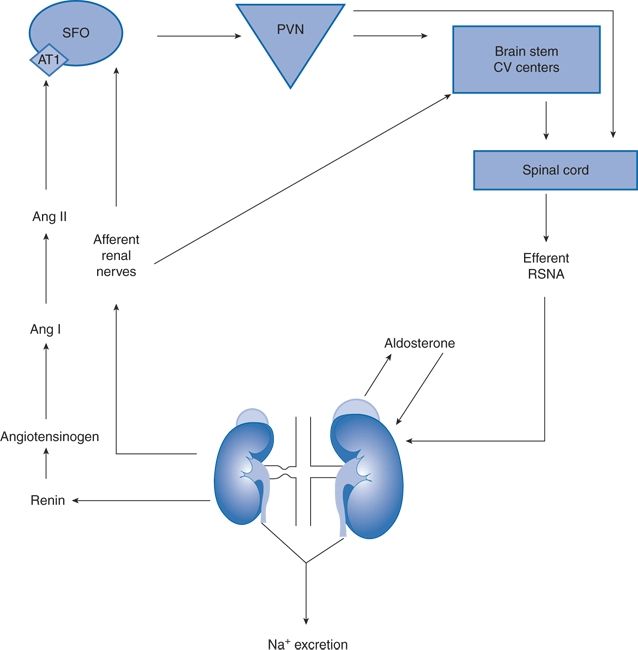
FIGURE 10-3 Synergistic interactions between the renal nerves and the renin–angiotensin–aldosterone system in the 2-kidney, 1-clip rat model of unilateral renal artery stenosis. Ang, angiotensin; SFO, subfornical organ; PVN, paraventricular nucleus; RSNA, renal sympathetic nerve activity (i.e., efferent renal nerves). (From Pradhan N, Rossi NF. Interactions between the sympathetic nervous system and angiotensin system in renovascular hypertension. Curr Hypertens Rev 2013;9:121–129.)
New Clinical Translational Research
Textor and coworkers have made a recent breakthrough in our understanding of the pathogenesis and progression of ARAS in patients using blood oxygen level–dependent renal magnetic resonance imaging (BOLD-MRI) (Textor & Lerman, 2013). As diagrammed in Figure 10-4, they propose three stages in the progression of ARAS:
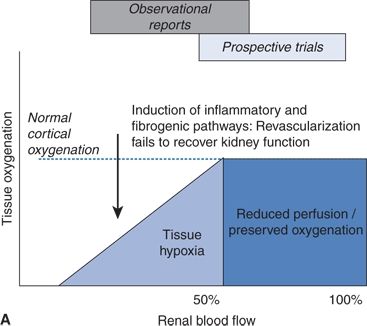
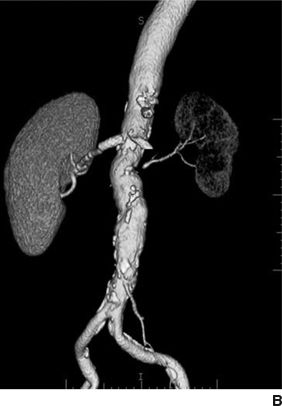
FIGURE 10.4 A: Schematic of tissue oxygenation within the kidney as blood flow falls beyond an ARAS. Despite moderate hemodynamic reduction in blood flow, medullary and cortical oxygenation is well tolerated in many patients. Beyond a certain limit, further reductions lead to overt cortical hypoxia, which is associated with inflammation and fibrosis that can persist despite restoration of main vessel patency. B:CT angiogram with a stent in the right kidney, with normal perfusion and function. The left kidney has lost volume, blood flow, and function that likely will not recover if renal patency is restored with stenting. (From Textor SC, Lerman LO. Renal artery stenosis: Medical versus interventional therapy. Curr Cardiol Rep 2013;15:409.)
- With mild–moderate/early- to mid-stage ARAS, renal blood flow is reduced by up to 50%, but renal tissue oxygenation is well preserved due mainly to a compensatory reduction in GFR and thus tissue oxygen demands (Gloviczki et al., 2010). These data were obtained in patients with unilateral moderate ARAS on stable ACEI- or ARB-based antihypertensive therapy. In such patients, renal artery stenting would not be expected to have any effect on renal perfusion, which is already normal. This may help explain the negative findings in the Angioplasty and Stenting for Renal Artery Lesion (ASTRAL) and Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) trials, which enrolled patients with mainly moderate rather than severe ARAS.
- With moderately severe/more advanced stage ARAS, renal cortical hypoxia occurs and begins to induce production of inflammatory cytokines and fibrotic pathways such as those involving transforming growth factor–beta (TGF-β). Renal artery stenting is most likely to improve or stabilize kidney function if the cortical hypoxia is caught soon enough—before irreversible damage to the microvasculature has occurred (Chrysochou et al., 2012b).
- With far advanced/end-stage ARAS, pruning of renal microvessels closes the window of opportunity to rescue renal function with stenting. Cortical blood flow can be restored, but it is too late to reverse renal inflammation/fibrosis and salvage the kidney (Saad et al., 2013). Early preclinical studies in pigs suggest the promise of stem cell therapy to rescue partially atrophic kidneys from such far-advanced ARAS (Textor & Lerman, 2013).
Thus, these elegant studies suggest that there is a very narrow middle-ground sweet spot in the natural history of ARAS where renal artery stenting will save the kidney. It is not clear if the same concept applies to effects of ARAS stenting on BP reduction, which are addressed later in the chapter.
CLASSIFICATION AND COURSE
The most common cause of RVHT is atherosclerotic stenosis of the main renal artery; most of the remaining cases are fibroplastic, but a number of both intrinsic and extrinsic lesions can induce RVHT (Table 10-2). The general features of the most common types of renal artery stenosis are listed in Table 10-3.
TABLE 10-2 Types of Lesions Associated with RVHT

aMore common in children.
TABLE 10-3 Features of the Two Major Forms of Renal Artery Stenosis
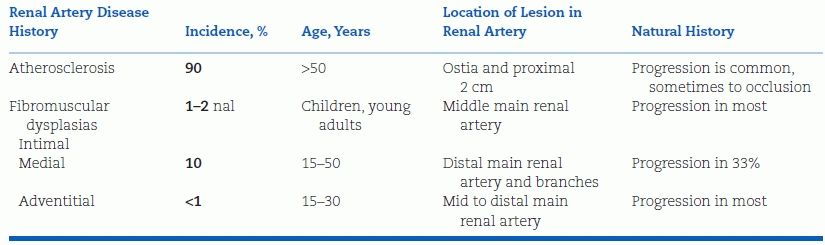
Atherosclerotic Lesions
As compared to patients with primary hypertension, patients with atherosclerotic RVHT are older and have higher systolic pressure, more extensive renal damage, and atherosclerotic vascular disease elsewhere. As a group, they also have more extensive left ventricular hypertrophy, ischemic heart disease, renal insufficiency, and, not surprisingly, lower probability of survival from these conditions (Sattur et al., 2013).
Natural History and Secular Trends
Since ARAS is a local manifestation of a systemic atherosclerosis, the relatively slow rate of progression of the renal lesion is countered by the more common cause of death by cardiovascular disease (Kalra et al., 2005). Medicare data show that ARAS patients have a threefold greater risk of death than do their non-ARAS counterparts (Kalra et al., 2010) and dialysis patients with ARAS have an annual mortality rate as high as 36% (Guo et al., 2007).
Medicare claims data show that between 1992 and 2004, the detection of ARAS increased threefold among patients over age 65 while the rate of revascularization peaked in 1999 and then started to decline (Kalra et al., 2010). A further (70%) decline in revascularization for ARAS has occurred in the United Kingdom (Health and Social Care Information Centre, 2014) with publication of 3 negative RCTs: Stent Placement and Blood Pressure and Lipid-Lowering for the Prevention of Progressive Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) (Bax et al., 2009), ASTRAL (Wheatley et al., 2009), and CORAL (Cooper et al., 2014).
Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD) is a nonatherosclerotic, noninflammatory disease of the renal arteries and other medium-sized arteries (especially the carotid artery) that can lead to stenosis, occlusion, dissection, and aneurysm (Olin et al., 2014).
Figures 10-5 and 10-6 show the two most common types of fibromuscular stenoses (Olin et al., 2014). The most common angiographic appearance in FMD is an artery that looks like a string of beads located in the mid and distal portion of the artery (see Fig. 10-5), in contrast to focal atherosclerotic stenosis located at the origin or proximal portion of the artery (see Fig. 10-4). The string of beads indicates multifocal medial fibroplasia. The next most common type is unifocal FMD characterized angiographically as a focal or tubular stenosis in the midportion of the artery (see Fig. 10-6). In the retrospective series of Savard et al. (2012) of 337 patients with established renal artery FMD, 82% were classified as multifocal and the other 18% as unifocal. Patients with unifocal versus multifocal lesions differed significantly in median age at diagnosis of FMD (30 vs. 49 years, unifocal vs. multifocal), hypertension (26 vs. 40 years), sex distribution (female:male ratio, 2:1 and 5:1), initial blood pressure (157/97 and 146/88 mm Hg), current smoking (50% and 26%), prevalence of unilateral renal artery lesions (79% vs. 38%), presence of kidney asymmetry (33% vs. 10%), renal revascularization procedures (90% vs. 35%), and hypertension cure rates in patients who underwent revascularization (54% vs. 26%).
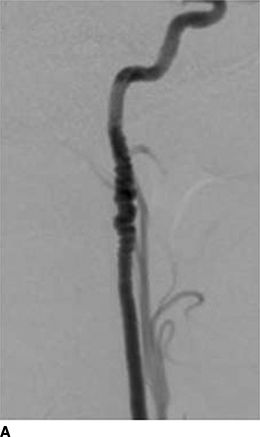
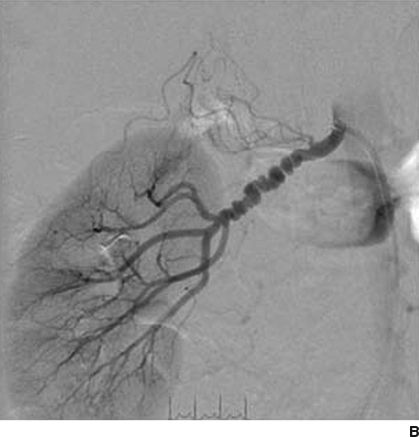

FIGURE 10.5 Multifocal (medial) FMD in the carotid (A) and renal (B) arteries. There are multiple areas of alternating stenosis and dilatation (string of beads), located in the mid to distal portion of the internal carotid and renal arteries. C: High-magnification photomicrograph showing a gap in the arterial media. In medial fibroplasia, there are alternating areas of thinned media and thickened fibromuscular ridges in which the arterial muscle is replaced by fibroplasia with loose collagen. (From Virmani R, Carter-Monroe N, Taylor AJ. Congenital anomalies and malformations of the vasculature. In: Creager MA, Beckman JA, Loscalzo J, eds. Vascular Medicine: A Companion to Braunwald’s Heart Disease. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2013.)
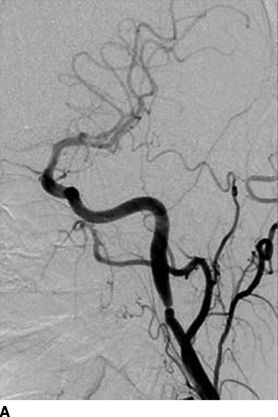
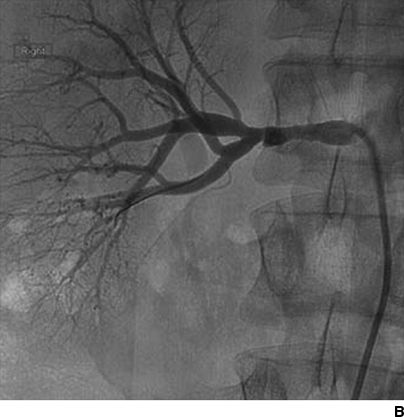
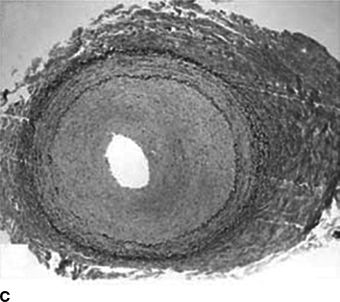
FIGURE 10-6 Focal FMD in the renal and internal carotid arteries. This angiographic pattern is most consistent with intimal fibroplasia. This can present with a concentric band (focal constriction) as shown in the right internal carotid artery (A) or the right renal artery (B). C: Histopathologic findings: concentric thickening of the intima. The media and adventitia are relatively normal. (A and B from Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129, 1048–1078. C from Virmani R, Carter-Monroe N, Taylor AJ. Congenital anomalies and malformations of the vasculature. In: Creager MA, Beckman JA, Loscalzo J, eds. Vascular Medicine: A Companion to Barunwald’s Heart Disease. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2013.)
A third type, termed perimedial FMD, occurs almost exclusively in children in whom it can cause hypertension and CKD (Olin et al., 2014). This unusual type of FMD was found in only 2 of 577 adult patients in the U.S. Registry for FMD (Olin et al., 2012).
As a historical aside, FMD was discovered serendipitously by Leadbetter and Burkland (1938). They cured severe hypertension in a 5-year-old boy by surgical removal of his solitary pelvic kidney and discovered the renal artery narrowing during pathologic examination of the postoperative specimen.
FMD involves not only the renal arteries but other medium-sized arteries, most notably the extracranial carotid, vertebral, mesenteric, and lower-extremity arteries. While aneurysms are most common with renal FMD, dissection is most common with carotid artery FMD occurring in 75% of cases (Olin et al., 2012).
The presenting symptoms and signs of FMD are shown in Table 10-4 (Olin et al., 2012). The most frequent presenting complaints are hypertension, headache, and pulsatile tinnitus—the last being described as a swooshing sound in the ears and a tip-off to the presence of carotid FMD. Only 5% of patients are asymptomatic at presentation; rarely, FMD is discovered during evaluation of an asymptomatic carotid or renal bruit or is an incidental finding on unrelated abdominal imaging.
TABLE 10-4 Consider the Diagnosis of FMD in the Following Circumstances

TIA, transient ischemic attack. (From Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: Results in the first 447 patients. Circulation 2012;125:3182–3190.)
The cause of FMD and explanation for the 10:1 female predominance is an enigma. Some studies suggest cigarette smoking as a risk factor, but others do not (Olin et al., 2014). Familial occurrence is only in 7% of cases (Olin et al., 2012). Few candidates have been proposed and none confirmed.
Other Causes
Of the myriad causes of RVH listed in Table 10-5, a few deserve additional comment.
TABLE 10-5 Interactive Mechanisms Underlying Hypertension and Kidney Injury in Atherosclerotic RAS
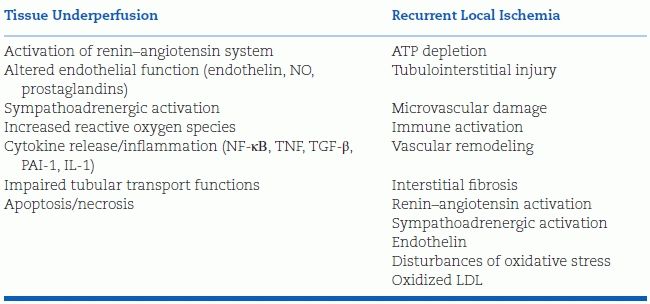
Modified from Textor SC. Atherosclerotic renal artery stenosis: Overtreated but underrated? J Am Soc Nephrol 2008;19:656–659.
Aneurysm
Aneurysms are common with multifocal renal FMD. Saccular aneurysms, usually at the bifurcation of the renal artery, may induce hypertension by various mechanisms. They rarely rupture and need not be ablated if less than 2.0 cm in diameter in the absence of symptoms or severe hypertension (English et al., 2004).
Emboli
Most commonly seen as a complication of angiography or vascular surgery, renal cholesterol emboli can induce renal failure or RVHT (Scolari et al., 2007). Cutaneous, ocular, and other visceral lesions are usually seen, and the diagnosis may be documented by biopsy of skin lesions.
Arteritis
Progressive aortic arteritis (Takayasu arteritis or pulseless disease) is seen infrequently in North America and Europe but is a common cause of RVHT in China, India, Japan, Mexico, and Brazil occurring in up to 60% of such patients (Chaudhry & Latif, 2013). If unrecognized, this can lead to renal failure and/or RVHT. It is seen mainly in children and young adults and is often associated with signs of chronic inflammation (Cakar et al., 2008) and requires stenting or surgical revascularization (Chaudhry & Latif, 2013).
Renal artery stenosis (or complete occlusion) is a common feature of antiphospholipid syndrome, a multisystem disorder characterized by arterial or venous thrombosis in association with antiphospholipid antibodies, namely anticardiolipin antibodies, lupus anticoagulant, and anti-beta2-glycoprotein I antibodies (Pons-Estel & Cervera, 2014). The syndrome and its associated renal artery stenosis may be primary or secondary to systemic lupus erythematosus or antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis of small- to- medium-sized blood vessels, with the antibodies being directed against cytoplasmic proteins (proteinase 3 [PR3] and myeloperoxidase [MPO]) expressed on the surface of neutrophils. The ANCA-positive vasculitides include granulomatosis with polyangiitis (formerly known as Wegener granulomatosis); Kawasaki disease; polyarteritis nodosa; microscopic polyangiitis; eosinophilic granulomatosis with polyangiitis (Churg-Strauss), IgA vasculitis (Henoch-Schönlein), and erythematosus; vasculitis related to rheumatoid arthritis; and Sjögren syndrome. Patients may enter into an acute, severe hypertensive phase, usually associated with markedly elevated plasma renin levels, likely reflecting intrarenal stenosis from multiple arteriolar lesions. The high-renin hypertension can sometimes be rather remarkably reversed by ACEI therapy (Tektonidou, 2009). The RVHT can be accompanied by renal vein thrombosis with nephrotic-range proteinuria (Pons-Estel & Cervera, 2014). Renal artery stenosis has been reported rarely in antiphospholipid syndrome with polycythemia vera (Zahra Ha-ou-Nou et al., 2014).
Aortic Dissection
Renal artery occlusion was found in nearly 20% of patients with distal aortic dissection (Rackson et al., 1990). The resulting renal ischemia and impaired renal function can be normalized by repair of the dissection (Verhoye et al., 2005).
CLINICAL FEATURES
Most patients with ARAS are older persons with hypertension and hyperlipidemia and often have clinically evident PAD, coronary disease, and/or cerebrovascular disease. As mentioned, the three most specific presentations of severe ARAS are drug-resistant hypertension, ischemic nephropathy, and flash pulmonary edema.
Hypertension
Clinical features suggestive of renovascular disease as the cause of hypertension are presented in Table 10-6 (Sattur et al., 2013). Some of these features were identified many years ago in a cooperative study involving 2,442 hypertensive patients, 880 with renovascular disease (Maxwell et al., 1972). Of the 880, 502 had surgery; of these, 60% had ARAS, and 35% had FMD. The clinical characteristics of 131 patients with surgically cured renovascular disease were compared to those in a carefully matched group with primary hypertension (Simon et al., 1972). Of the clinical features more common in patients with RVHT, only an abdominal bruit was of clear discriminatory value, heard in 46% of those with renovascular but in only 9% of those with primary hypertension. The bruit was heard over the flank in 12% of those with RVHT and in only 1% of those with primary hypertension. Most systolic bruits are innocent, but systolic–diastolic bruits in hypertensives are suggestive of RVHT (Turnbull, 1995).
TABLE 10-6 Clinical Clues for RVHT

Hypertensive Heart Disease
In patients with RVHT and relatively preserved renal function, BP is more difficult to control than in patients with primary hypertension, and echocardiograms show a greater degree of concentric left ventricular hypertrophy with more diastolic and systolic dysfunction (Khangura et al., 2014). The combination of pressure overload and neurohormonal activation with cardiovascular inflammation conspire to aggravate hypertensive heart disease, which sets the stage for episodes of acute (“flash”) pulmonary edema.
Hyperaldosteronism
Patients with RVHT occasionally have profound secondary aldosteronism with hypokalemia due to urinary potassium wasting but low serum sodium unlike the high serum sodium seen in primary aldosteronism (Agarwal et al., 1999)—all reversed with relief of RVHT. The explanation for the association is unknown.
Ischemic Nephropathy
Beyond hypertension, the second most common clinical presentation of renal artery stenosis is ischemic nephropathy, which is estimated to be the cause of ESRD in at least 5% of patients entering chronic dialysis (Levin et al., 2007).
Patients with ischemic nephropathy may be difficult to distinguish from the larger number with primary hypertension or primary renal parenchymal disease that progresses into renal failure. The possibility of bilateral renovascular disease should be considered in the following groups (Sattur et al., 2013):
- Young women with severe hypertension, in whom fibroplastic disease is common.
- Older patients with extensive atherosclerotic disease who suddenly have a worsening of renal function.
- Any hypertensive patient who develops rapidly progressive renal failure without evidence of obstructive uropathy.
- Patients in whom renal function deteriorates abruptly and progressively after treatment with an ACEI or ARB.
- Hypertensives who develop multiple episodes of acute pulmonary edema.
Flash Pulmonary Edema
Pickering et al. (1988) was first to implicate ARAS as a reversible cause of recurrent sudden (i.e., “flash”) pulmonary edema (Fig. 10-7) (Sarkodieh et al., 2013). Of 11 patients with ARAS and multiple episodes of pulmonary edema, 7 had stenosis of both renal arteries, 2 had stenosis of the artery to a solitary kidney, and 2 had unilateral stenosis with an intact contralateral kidney. Successful revascularization (by angioplasty in eight and surgery in three) improved BP and renal function, and virtually eliminated pulmonary edema. In their second series of 55 consecutive patients with advanced CKD and ARAS, pulmonary edema occurred in 23%.
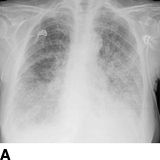
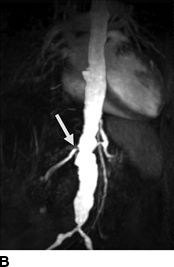
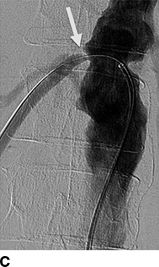
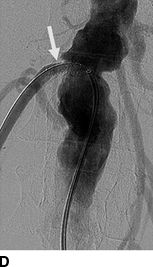
FIGURE 10-7 A patient with a single functioning kidney who presented with acute shortness of breath. A: Chest radiograph shows flash pulmonary edema. B: Renal magnetic resonance angiogram (MRA) shows right renal artery stenosis (arrow). C: Digital subtraction angiography (DSA) confirms severe short segment stenosis of the right renal artery origin (arrow). D: A 6- × 17-mm stent (arrow) was inserted producing a good angiographic result. (From Sarkodieh JE, Walden SH, Low D. Imaging and management of atherosclerotic renal artery stenosis. Clin Radiol 2013;68:627–635.)
A recent observational study of 467 with ARAS treated at the Manchester Academic Health Sciences Centre found that the 37 patients presenting with flash pulmonary edema had a threefold increase in CV events and a twofold increase in death than did patients without this presentation (Ritchie et al., 2014). In this subgroup, stenting rather than medical treatment (according to physician preference) was associated with a 60% reduction in mortality.
Other Scenarios
Hypertension After Renal Transplantation
As described in Chapter 9, patients who develop severe hypertension after renal transplantation should be evaluated for stenosis of the renal artery. Posttransplant stenosis have been reported in 2% about of renal allografts in experienced transplant centers and generally have an excellent outcome with renal angioplasty (Su et al., 2012).
Impact of ARAS on Outcomes After Open Heart Surgery
Renal artery stenosis may be one cause of acute kidney injury after open heart surgery particularly in older patients undergoing coronary bypass grafting, aortic valve replacement, or aortic aneurysm repair. However, in a recent Cleveland Clinic series of 714 patients undergoing open heart surgery, no association was found between ARAS (detected by duplex ultrasonography in 29% of the patients) and surgical outcomes (Philip et al., 2014). While older patients and those undergoing descending aortic grafting were at high risk for postoperative acute kidney injury, the risk was similar in those with and without ARAS.
DIAGNOSTIC TESTS
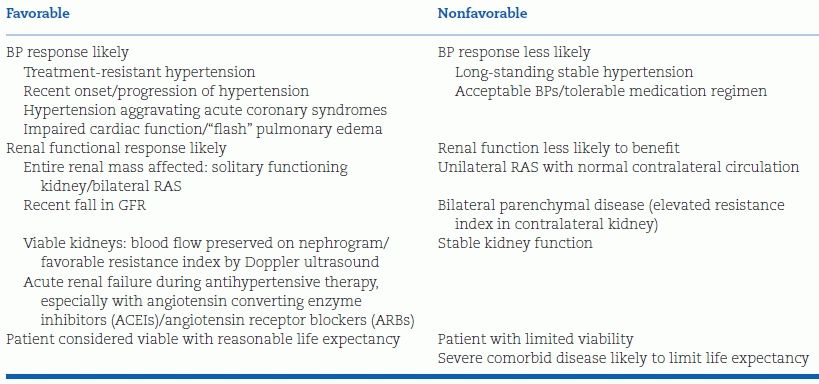
Before any tests are performed to diagnose RVHT, the clinician should consider whether, if renal stenosis is present, revascularization would be indicated to provide likely benefit despite the possible complications (Textor & Lerman, 2013). As listed on the right side of Table 10-7, for those patients with stable renal function and long-standing stable hypertension that is responsive to easily tolerated antihypertensive drugs, revascularization would likely provide no benefit; therefore, no tests should be performed. On the other hand, in those with one or more factors that make a favorable response to revascularization more likely, listed on the left side of Table 10-6, testing should be performed to define the extent of renovascular disease and estimate its functional significance. In those with a high likelihood of reversible RVHT, invasive angiography and, if significant stenosis is seen, angioplasty with stenting are appropriate.
TABLE 10-7 Factors Indicative of Response to Revascularization for Atherosclerotic RVHT
Imaging for suspected clinically important ARAS can be divided into three steps: (1) Noninvasive functional imaging, (2) computed tomography (CT) or MR angiography, and (3) invasive digital subtraction angiography (DSA).
Noninvasive Functional Imaging
As mentioned, there are no perfect tests to predict with certainty a favorable response to renal artery stenting. The available noninvasive tests are Doppler ultrasound with resistive index and nuclear medicine scans (renography).
Duplex Ultrasonography with Resistive Index
A Doppler flow signal from a normal renal artery and from a stenotic artery are shown in Figures 10-8 and 10-9. The conventional criterion for diagnosis of renal artery stenosis is a greater than 3.5-fold greater flow velocity at the site of the stenosis (postobstructive jet) in the proximal main renal artery than in the unobstructed aorta (Soulez et al., 2000). However, it is easy to underestimate the peak velocity if the Doppler signal is not directly in line with the jet or obscured by overlying soft tissue and bowel gas. A more sensitive but semiquantitative method is to search for a diminished (“parvus et tardus”) flow velocity waveform in the intrarenal arteries distal to the stenosis (Fig. 10-9). The sensitivity is 75% and specificity 90% when compared with the gold standard of DSA (Hashemi et al., 2011).
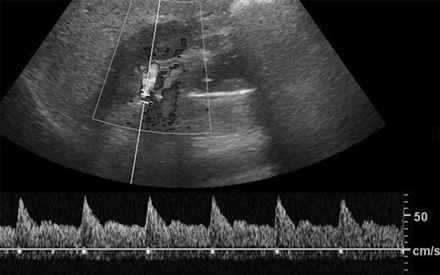
FIGURE 10-8 Duplex Doppler ultrasound of the main renal artery in a healthy adult, showing a sharp systolic upstroke and peak systolic velocity of 70 cm/s. (From Sarkodieh JE, Walden, SH, Low D. Imaging and management of atherosclerotic renal artery stenosis. Clin Radiol 2013;68:627–635.)
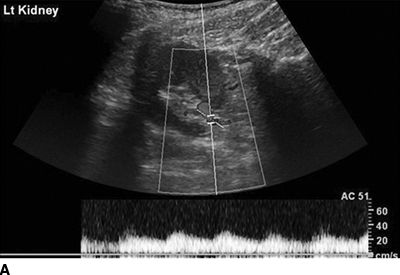
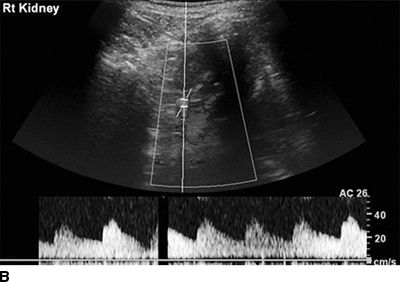
FIGURE 10-9 Duplex Doppler ultrasound of the distal left and right renal arteries in a patient with unilateral renal artery stenosis. A: Left kidney: tardus–parvus waveform indicating renal artery stenosis. B: Right kidney of the same patient showing a normal Doppler waveform. (From Sarkodieh JE, Walden SH, Low D. Imaging and management of atherosclerotic renal artery stenosis. Clin Radiol 2013;68:627–635.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








