Figure 79-1 Pelvic magnetic resonance imaging scan with and without gadolinium. A, Oblique coronal T1-weighted image (T1WI) demonstrates isointense enlargement of the right S1 and S2 nerve roots. B, Fat-saturated T2-weighted image (T2WI) demonstrates the enlarged right S1 and S2 nerve roots to be hyperintense. C, Fat-saturated T1WI after gadolinium demonstrates asymmetric enhancement. D, Axial T1WI inferior to the sacrum demonstrates asymmetric enlargement of the right sciatic nerve (black arrow) when compared with the normal left side (white arrow).
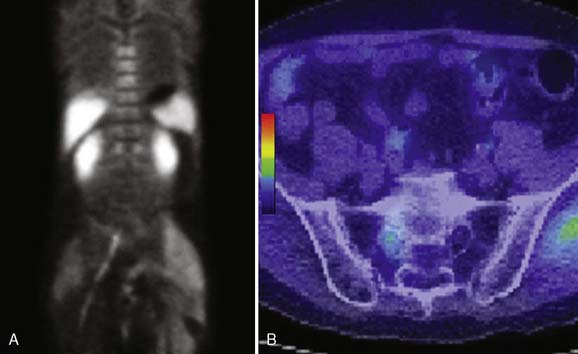
Figure 79-2 11C-choline positron emission tomography (PET) scan. A, Coronal image through the sacral plexus demonstrates increased radiotracer accumulation extending from S1 into the right sciatic nerve. B, Fused computed tomography and PET axial image demonstrates asymmetric uptake in the enlarged right S1 nerve root. Normal muscular activity is seen in the left gluteal muscle. The myxoma located at the level of the trochanters on the left (not shown on this image) did not demonstrate increased uptake.
A right L5-S1 laminoforaminotomy was performed to obtain an S1 nerve root biopsy. Pathology showed PSA-positive adenocarcinoma. The patient was then started on bicalutamide (nonsteroidal antiandrogen) and underwent local irradiation to the lumbosacral plexus. At 9-month follow-up by correspondence, his pain was markedly improved and imaging of his spine demonstrated stable findings.
CONCLUSIONS
Direct invasion of the lumbosacral plexus, although rare, is becoming a well-recognized entity. Still, the most common cause of neoplastic LSP is colorectal cancer.1 As discussed in Chapter 14, we also believe that perineural spread, well known in head and neck cancer,2 also explains LSP in prostate carcinoma cases. Tumor cells can spread in a retrograde manner through S2-4 nerve roots, then anterograde through the sciatic nerve.3
Painful LSP is a difficult diagnosis. Some patients are misdiagnosed and may even undergo lumbar spine surgery before the correct diagnosis is made. Keys to the diagnosis include a clinical picture out of proportion to lumbar MRI findings. Atypical symptoms that should raise suspicion for neoplastic plexopathy include nocturnal, deep aching or burning pain, nonmechanical pain and significant motor deficits that include proximal muscle groups. Other patients who have undergone radiation therapy may be assumed to have radiation plexopathy. The distinction between radiation and neoplastic LSP may be difficult. Painless, bilateral weakness is often present in patients with radiation-induced plexopathy.4
Detailed history, clinical examination and electrodiagnostic studies can establish the localization of the neurologic deficits to the lumbosacral plexus. The history of prostate carcinoma and a rising PSA should also alert clinicians to the possibility of metastatic prostate carcinoma as a specific cause. As illustrated in this case, high-resolution MRI and PET play an extremely important role in localizing the lesion. If the MRI and PET imaging are convincing, biopsy may be deemed unnecessary. However, in many cases, the definitive diagnosis can be obtained only with biopsy. The most abnormal area seen on imaging that is most accessible and safe can be targeted for biopsy. Appropriate treatment consisting of chemotherapy and radiation therapy can then be initiated.
Imaging of the lumbosacral plexus is relatively complex. If possible, it should be performed on a higher field 3T MRI scanner, using multichannel surface coils, and the smallest field of view (FOV) possible to yield the highest resolution imaging. Axial coverage should extend from L4 through both greater trochanters employing a FOV of 26 to 34 and thin slices (<5 mm) to cover the entire lumbosacral plexus and proximal sciatic nerve. Oblique coronal images through the sacrum should extend from the back of the sacrum through the sciatic notch with a FOV 24-28 and slice thickness of less than 4 mm. Sequences should include T1-weighted images (T1WI) without fat saturation for anatomic detail, fat-saturated T2WI for intrinsic neural signal evaluation, and fat-saturated postgadolinium T1WI in both planes. If isolated lesions are detected, smaller FOV and thinner slices can be obtained through that region to aid in surgical resectability. 3T MRI of the lumbosacral plexus in patients with perineural tumor spread from prostate cancer typically reveals moderately enhancing, T2 hyperintense nodular or smooth enlargement of the lumbosacral plexus that can then spread anterograde along the sciatic nerve.
The differential diagnosis based on imaging characteristics may include neurogenic tumors such as a schwannoma, lymphoma, metastasis, or neurosarcoid. It is in these cases that PET/CT aids in the diagnosis. Owing to the fact that many of these entities can be very similar in appearance on MRI, PET/CT can aid in differentiating them. In patients with neoplastic involvement, the nerve roots can have smooth or nodular enhancement and generally more than one root of the plexus are involved either continuously or in a skip pattern. Lymphoma involving the lumbosacral plexus is characterized by multiple smooth enlarged homogeneously enhancing nerve roots. Lymphadenopathy elsewhere in the retroperitoneum or abdomen may be helpful but is not always present. Neurosarcoid can have one or multiple nerve roots involved; other systemic findings such as mediastinal lymphadenopathy, interstitial lung involvement, or lacrimal and salivary gland findings may help differentiating this entity from neoplasm. Laboratory data such as angiotensin-converting enzyme levels may also aid establishing the diagnosis. Radiation plexopathy, less commonly seen in prostate cancer due to dose and localization of radiation in these patients, is more frequent in other radiated neoplasms such as rectal carcinoma or gynecologic malignancies. The MRI appearance of radiation-induced plexopathy is variable but usually shows MRI features of fibrosis consisting of diffuse smooth thickening of the nerves with variable degree of gadolinium enhancement. The radiation changes also tend to be symmetric, which helps in differentiating radiation-induced plexopathies from neoplastic plexopathies.
Although there are no studies describing results of PET scans in patients with LSP due to prostate cancer, we can draw some useful information from data in patients with non-neural metastatic prostatic cancer. In the latter group, 11C-choline PET/CT has recently been proven as a reliable imaging modality in initial staging of prostate cancer and in restaging patients who have undergone a radical prostatectomy and have a rising PSA.5 It has been shown to be more sensitive than 18F-fluorodeoxyglucose PET.6 Recently, 11C-choline PET/CT has been shown to have an overall sensitivity of 93% and a positive predictive value of 80% in the detection of recurrence in prostate cancer patients with biochemical recurrence (increasing PSA level) after radical prostatectomy.7 The rationale of 11C-choline PET scanning for tumor imaging is based on the incorporation of choline as phosphatidylcholine into the cell membrane of neoplastic cells, which have increased turnover in comparison to normal cells. Normal uptake is seen in the salivary glands, thyroid gland, pituitary, choroid plexus, liver, spleen, kidneys, bowel and pancreas. The differential diagnosis for uptake of 11C-choline along the lumbosacral plexus is broad including metastasis, neurosarcoid, lymphoma, and inflammatory lesions. However when the patient’s history, physical examination findings, and cross-section imaging are combined with the 11C-choline PET/CT, the diagnosis can be made with more certainty, particularly in the setting of metastatic prostate cancer. Data from non-neural metastases showed that the detection rate are dependent on the serum PSA value.7,8 However, metastasis has been detected with PSA as low as 0.5 ng/mL and is considered useful for restaging of prostate cancer patients with rising PSA even at levels below 1.5 ng/mL.7
High-resolution imaging, as demonstrated in this paper, is an important adjunct for the diagnosis and management of patients with lumbosacral plexopathy of unknown etiology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








