Seizures and Epilepsies in the Preterm and Term Neonate
Emma Laureta
Eli M. Mizrahi
Solomon L. Moshé
NEONATAL SEIZURES
Historical Aspects
Initial investigations and attempts to characterize and classify neonatal seizures began in the 1950s and 1960s, as neonatal electroencephalography (EEG) began to emerge and well before video-EEG recordings became established as diagnostic tools (1, 2, 3 and 4). Early investigators relied on clinical observation or observation supplemented by interictal EEG recordings (5,6). Later, bedside EEG recordings of infants who were experiencing seizures combined with cinematography, improved correlation of observed clinical phenomenon with electrographic activity (7, 8, 9 and 10).
These pioneering French investigators observed that seizures in the neonate had unique clinical features that distinguished them from seizures generated by more mature brains. For example, generalized tonic-clonic seizures were found not to occur (5,10, 11 and 12). The more common focal clonic seizures were often asynchronous if they occurred bilaterally and did not spread in typical Jacksonian sequence (2,8, 9 and 10).
Also, in the 1950s and 1960s, paroxysmal phenomenon with minimal motor manifestations began to be recognized as clinical seizures. These included paroxysmal ocular and oro-buccallingual movements, repetitive limb movements resembling pedaling, rowing or swimming movements, changes in skin color, respiration, and other autonomic changes (5,7). They were initially characterized as “anarchic or atypical” and later various other terms have been used to describe these seizures including “slight,” “minimal,” “subtle,” or “motor automatisms” (9,10,13, 14, 15 and 16). Many of the so-called subtle seizures were subsequently found not to be associated with simultaneous electrographic discharges. This was also observed to be true for other seizures, such as those characterized by generalized tonic posturing. Over the years, this has led to controversy over their pathophysiology. Observing that these events were similar to certain reflex behaviors, Mizrahi and Kellaway postulated that these were primitive brainstem reflexes “released” by forebrain depression, rather than true epileptic events (17,18).
These observations and controversies have led to the evolution of current classification systems of neonatal seizures, which are primarily based on clinical semiology, electroencephalographic features, the temporal relationship of clinical and EEG features, and presumed pathophysiology. They have also led to several issues regarding diagnosis and management of neonatal seizures especially those unaccompanied by prominent clinical manifestations.
Terminology
In the term infant, the neonatal period is defined as the first 28 days of life. In the preterm infant, this period extends to the 44 completed weeks gestational age. It is a period of increased vulnerability to anoxic and metabolic stress, and most seizures during this period are acute reactive seizures, as opposed to manifestations of neonatal epilepsy—although the latter may occur with early neonatal seizures being the first of a chronic condition (19). However, the term “epileptic” is used in current classification systems to denote presumed underlying pathophysiology—seizures generated by hypersynchronous cortical neuronal discharges as demonstrated by temporally related EEG changes. This is in contrast to “nonepileptic” seizures that occur without any EEG correlate and are of unclear pathophysiology, most likely based in reflex physiology. Focal clonic, focal tonic events, some myoclonic events and spasms are most often epileptic. Generalized tonic posturing and motor automatisms are often purely clinical. Seizures recorded in the absence of clinical activity are termed “electrical only or electrographic seizures.” These may occur in infants not treated with antiepileptic drugs (AEDs) with encephalopathy or those with electroclinical seizures who have been treated with AEDs with subsequent “decoupling” of electrical and clinical manifestations (17).
Epidemiology
Reported incidence of neonatal seizures range from 1.8 to 5.5 per 1000 newborns (20, 21, 22 and 23). The National Collaborative Perinatal Project, which studied infants of 54,000 pregnant women between 1959 and 1966, reported an incidence of 5.1/1000 live births (24). More recent population-based studies in Fayette County, Kentucky; Newfoundland, Canada; and Harris County, Texas have reported lower incidences rates, between 1.8/1000 and 2.5/1000 live births (20, 21, 22 and 23). Differences in target population, periods of surveillance, methods used in case identification, and use of clinical versus clinical and EEG criteria in the identification of cases likely account for the variability in reported rates (25). The incidence of neonatal seizures is higher in premature and low birth weight infants, as well as infants cared for in intensive care units (23). Neonatal seizures are most common in the first week of life (20,26).
Pathophysiology
Seizures occur more frequently in the neonatal period than at any other time in life. Basic science research has shown various factors underlie this increased susceptibility to seizures, based primarily on relatively enhanced excitation in the immature brain
(27, 28, 29, 30, 31 and 32). For example, in the immature brain, potassium tends to accumulate in the extracellular space, secondary to decreased Na+, K-ATPase activity, and immature enzyme systems. This leads to the development of a hyperexcitable state and decreased seizure threshold (27). Excitation may also be enhanced by a relative increase in the density of glutamate receptors and NMDAgated channels. On the other hand, synapses and receptors for inhibitory neurotransmitters may be less abundant in the developing brain. Also, GABA, the main inhibitory neurotransmitter in the CNS, may have excitatory functions early on (33, 34 and 35). In animal models, the substantia nigra pars reticularis, which in adults is involved in the control of seizures, has been shown to amplify seizures in immature animals. These and other factors, not only lower the overall seizure threshold, but likely, also allow seizures to spread more readily (25).
(27, 28, 29, 30, 31 and 32). For example, in the immature brain, potassium tends to accumulate in the extracellular space, secondary to decreased Na+, K-ATPase activity, and immature enzyme systems. This leads to the development of a hyperexcitable state and decreased seizure threshold (27). Excitation may also be enhanced by a relative increase in the density of glutamate receptors and NMDAgated channels. On the other hand, synapses and receptors for inhibitory neurotransmitters may be less abundant in the developing brain. Also, GABA, the main inhibitory neurotransmitter in the CNS, may have excitatory functions early on (33, 34 and 35). In animal models, the substantia nigra pars reticularis, which in adults is involved in the control of seizures, has been shown to amplify seizures in immature animals. These and other factors, not only lower the overall seizure threshold, but likely, also allow seizures to spread more readily (25).
Long-term consequences of neonatal seizures on brain development are unclear and likely affected by multiple factors, such as seizure burden, age of the infant when seizures occur, and underlying etiology. Despite the increased susceptibility to seizures, studies using animal models/rodents provide evidence to suggest that the immature brain is more resistant to seizureinduced hippocampal injury (28). However, in rats with a preex-isting lesion, the risk of status epilpeticus (SE)-induced injury is significantly elevated (36). Also severe recurrent seizures can lead to impaired synaptogenesis, myelination, and brain growth. Galanopoulou has shown that three SE in P4-6 rat pups may upregulate the expression of the chloride cotransporter KCC2 in immature neurons with still depolarizing GABAA receptor responses, which may prevent the maintenance of the GABAmediated effects on cell maturation (37,38). This effect differs from those described in adults and may therefore underlie and partially explain the age-specific consequences of SE.
There are also studies that suggest that exposure to currently available AEDs used to control seizures during this period, may have similar detrimental effects causing increased apoptosis or even on occasion promote seizures. In each case, the cost benefit ratio of the effects of seizures or AEDs must be determined, although ideally age-specific and even gender-specific treatments should be developed (see the following section).
Clinical and EEG Features of Neonatal Seizures
Characterization and Classification of Neonatal Seizures
Traditional classification schemes for seizures have been difficult to apply to neonatal seizures that are generally fleeting events occurring in infants with limited repertoire of normal behavior to begin with. More than one seizure type can occur in a particular infant.
The two most widely accepted classification schemes for neonatal seizures are by Volpe and by Mizrahi and Kellaway Each characterizes seizures based on semiology of the most prominent movement. In Volpe’s classification scheme, these neonatal seizures are classified as (i) clonic, (ii) tonic, (iii) myoclonic, and (iv) subtle (15,39). Mizrahi and Kellaway’s classification scheme, developed from findings on their EEG-polygraphic monitoring studies, not only considers semiology, but emphasizes the association of clinical behaviors with presumed pathophysiology based on the presence or absence of simultaneous EEG changes (16,17). In their classification scheme, neonatal seizures are broadly categorized as (i) epileptic events—when associated with consistent electrocortical signature, (ii) nonepileptic events—when unaccompanied by time-synchronized EEG changes, and (iii) electrical seizures—when EEG seizure discharges are not associated with obvious clinical manifestations (17).
The types of seizures found to be most consistently associated with EEG changes include focal clonic, focal tonic, some myoclonic seizures, and spasms. Seizures not consistently associated with an electrographic signature include generalized tonic, some myoclonic, and motor automatisms. Mizrahi and Kellaway used the term “motor automatisms” to describe seizures consisting of a variety of paroxysmal behaviors such as oral-buccal-lingual movements, ocular signs, progression movements, and complex purposeful movements. Each clinical seizure type and their associated EEG abnormalities are described in detail below.
Focal Clonic: These seizures consist of rhythmic, usually slow (approximately one to three jerks per second) repetitive movements of the face, proximal or distal arm or leg muscles, or axial structures. The movements may be unifocal (confined to one part of the body); hemiconvulsive (involving one side of the body); or multifocal (involving multiple body parts on both sides). Clonic seizures that involve both sides of the body simultaneously are usually asynchronous unlike true generalized clonic seizures in older individuals. Seizures may alternate between sites within a particular seizure, or may migrate from one site to another, not necessarily spreading in traditional Jacksonian sequence. Focal clonic seizures are usually associated with focal brain lesions such as cerebral infarctions but can also occur with more diffuse neuropathologic processes. By far, they are the type of seizures most consistently associated with electrographic correlate.
Focal Tonic: Sustained posturing of a limb, or unilateral flexion of the trunk characterize these seizures that can also be accompanied by sustained conjugate deviation to one side. Like focal clonic seizure, tonic seizures are usually associated with synchronized EEG discharges. They occur less frequently than focal clonic seizures.
Generalized Tonic: These seizures are characterized by sustained bilateral extension or flexion of the limbs or trunk, resembling decerebrate or decorticate posturing. Unlike their more focal counterpart, these seizures are generally not associated with EEG correlate and are classified as nonepileptic. Because they tend to occur in association with other automatic behavior in obtunded infants with diffuse central nervous pathologic processes, Mizrahi and Kellaway proposed that such seizures are “brainstem release phenomenon” from disinhibition of forebrain structures in clinically depressed infants.
Myoclonic: Irregular, erratic twitching, or contractions of muscle groups involving the face, limbs, or trunk are termed myoclonus. These can be either epileptic or nonepileptic. Myoclonic movements associated with EEG changes are also called “cortical myoclonus,” while myoclonus with no EEG
correlate is termed “sub-cortical myoclonus.” The latter term implies that more caudal structures in the brainstem or spinal cord responsible for generating the movements. The seizures can be focal, usually involving muscles of one upper extremity; or they may be generalized or multifocal. Generalized myoclonus consists of bilateral symmetric flexion jerks of the limbs or trunk. Multifocal or “fragmentary” myoclonus is characterized by brief asynchronous twitching of different muscle groups. Of the three types, fragmentary myoclonus is the type least associated with EEG changes, while two thirds of generalized myoclonus have EEG correlate.
correlate is termed “sub-cortical myoclonus.” The latter term implies that more caudal structures in the brainstem or spinal cord responsible for generating the movements. The seizures can be focal, usually involving muscles of one upper extremity; or they may be generalized or multifocal. Generalized myoclonus consists of bilateral symmetric flexion jerks of the limbs or trunk. Multifocal or “fragmentary” myoclonus is characterized by brief asynchronous twitching of different muscle groups. Of the three types, fragmentary myoclonus is the type least associated with EEG changes, while two thirds of generalized myoclonus have EEG correlate.
Spasms: Spasms characterized by bilateral flexor, extensor, or mixed flexion and extension contractions of limb and truncal muscles occur rarely in the neonate. Like spasms in older infants, they can occur in clusters and can be more frequent upon awakening. On EEG, they are associated with a generalized attenuation of the background or a high-voltage slow wave. Spasms are not included in Volpe’s classification scheme.
Motor Automatisms: Also called “subtle seizures,” paroxysmal changes in behavior or autonomic functions with minimal motor manifestations occur frequently in the newborn. These consist of various irregular and disconjugate ocular movements, eye-opening, chewing, oral-buccal movements, and peculiar extremity movements such as pedaling, stepping, boxing, swimming movements. Mizrahi and Kellaway found these behaviors to be inconsistently associated with EEG seizure and classify them as nonepileptic. However, similar behaviors, in preterm, and some full term infants have been demonstrated by other investigators to be ictal phenomena associated with simultaneous abnormal EEG discharges (40). Similarly, in older individuals, paroxysmal automatic behaviors are known to occur without surface EEG correlate as manifestations of psychomotor seizures or postictal behavior (41). Studies in neonatal animals have also shown that epileptiform discharges can occur in deep limbic structures with minimal or no propagation to the cortical surface (30). These observations raise the possibility that subtle seizures in neonates may be true epileptic events with generators in deeper limbic structures if concomitant electrographic seizure activity is the only criteria. These observations, however, do not exclude the possibility that some of these events behavior may be reflex behaviors.
Autonomic Signs: A variety of paroxysmal changes in autonomic activity such as alterations in breathing, heart rate, blood pressure, salivation, sweating, color changes, have also been described as manifestations of subtle seizures (42, 43 and 44). However, in isolation, they rarely occur as epileptic phenomena. When they are accompanied by ictal EEG correlates, they occur in association with other clinical behavioral or motor phenomenon. In a study by Watanabe et al., 14 of the 21 infants with apneic seizures with electrical EEG correlate exhibited other subtle phenomenon such as eye-opening, “staring,” deviation of the eyes and mouth movements (45). Bradycardia was also found to be more common in “convulsive apnea” versus “nonconvulsive” apnea in another study (46).
Ictal EEG Correlates
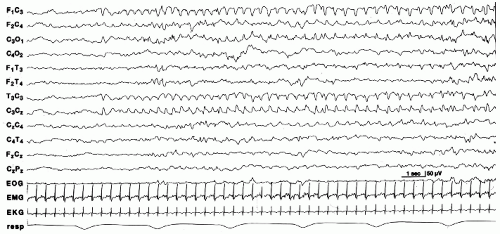
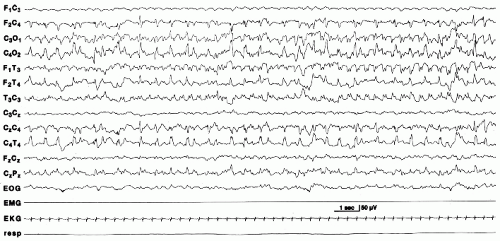
Electrical seizures are more common in term than in preterm infants. When they occur in infants less than 34 to 35 weeks, they are shorter in duration (23). Onset is usually focal, usually in the central and temporal regions (Fig. 25.1). Less common sites of origin are the frontal and occipital regions. Seizures can arise from more than one area, propagating asynchronously from different foci (Fig. 25.2). Frequency, morphology, and voltage of the discharge may vary within the same seizure, or from one seizure to the next in a given infant. The seizure discharge can consist of repetitive spikes, sharps or slow waves, or a combination of different waveforms. Seizures may remain localized to a specific area, slowly spread to involve contiguous regions, abruptly involve one of hemisphere, or migrate to the other hemisphere (25).
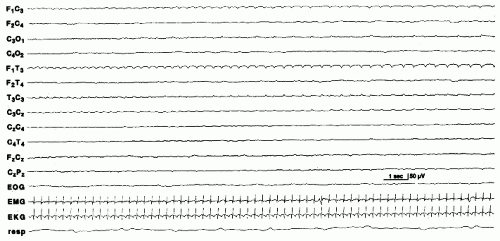
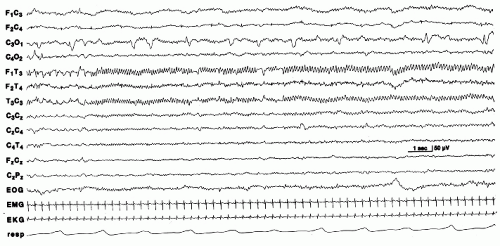
Electrographic seizures in the absence of clinical activity in severely encephalopathic neonates are characterized by background EEG activity that is usually depressed and undifferentiated. Some are lower in voltage, longer in duration, and show little tendency to spread or change. These have been referred to as “seizure discharges of the depressed brain” (26) (Fig. 25.3). Alpha seizure discharges are characterized as paroxysmal 8 to 12 Hz alpha activity in the central or temporal regions in encephalopathic newborns and suggest a poor prognosis (45,47,48) (Fig. 25.4).
When neonates are treated for seizures with antiepileptic medications, persistent electrical seizure activity can be found
long after clinical seizures have stopped. This “decoupling” of electrical from clinical activity, first described by Mizrahi and Kellaway, has been demonstrated by other investigators (17). In a study by Clancy and coworkers, 79% of 393 electrographic seizures recorded were not accompanied by clinical seizure activity. Seizures were the result of diverse etiologies and most infants had received one or more AED prior to the EEG recordings (49). Similar seizures without clinical correlates were also noted by Scher, Bye and Flanagan, and Murray to occur frequently (23,40,50).
long after clinical seizures have stopped. This “decoupling” of electrical from clinical activity, first described by Mizrahi and Kellaway, has been demonstrated by other investigators (17). In a study by Clancy and coworkers, 79% of 393 electrographic seizures recorded were not accompanied by clinical seizure activity. Seizures were the result of diverse etiologies and most infants had received one or more AED prior to the EEG recordings (49). Similar seizures without clinical correlates were also noted by Scher, Bye and Flanagan, and Murray to occur frequently (23,40,50).
Interictal EEG Correlates
Although the documentation of simultaneous EEG discharge during clinical events is useful in the diagnosis of seizures in the neonate, the finding of sharp-wave transients on interictal records does not aid in the diagnosis of seizures, or predict epileptogenicity. Unlike in adults, transient sharp waves, frequently found in the central and temporal regions are a nonspecific finding that can be seen in pathologically or as normal finding in term and preterm infants (26,51,52). Early studies suggested that positive rolandic sharp waves were most specific for intraventricular hemorrhage (53, 54 and 55), but subsequently have been more closely associated with periventricular leukomalacia.
However, in the setting of neonatal seizures, assessment of the interictal EEG background is still clinically useful. It can suggest possible etiologies for the seizures, help in determining an infant’s degree of cerebral dysfunction, risk for persistent seizures, and prognosis for long-term outcome.
EEG findings correlating with diffuse cerebral dysfunction include a depressed undifferentiated background, suppression-burst pattern, multifocal sharp transients, and dysmaturity. These abnormalities, although not typically etiologic specific, suggest etiologies which result in generalized or multifocal brain injury, such as hypoxia, acute metabolic disturbances, inborn errors of metabolism, infection, and bilateral brain hemorrhage. On the other hand, persistent asymmetries of the background and persistently lateralized sharp transients may indicate focal structural brain abnormalities such as focal cerebral infarction or areas of dysgenesis as underlying etiologies. The absence of focal features does not rule a focal structural pathology (25).
The EEG obtained within the first 24 hours can help in determining prognosis and risk for further seizures. Prospective studies in infants presenting with seizures in the neonatal period have found that the background EEG can be used for prognostic purposes to predict survival and long-term outcome (25,56, 57 and 58). A normal EEG suggests a good prognosis while the
presence and persistence of diffuse abnormalities on serial EEGs suggest poor outcome.
presence and persistence of diffuse abnormalities on serial EEGs suggest poor outcome.
EEG background may also predict persistent seizures in the acute period as well as in the postneonatal period. Laroia and coworkers, studying 51 infants at risk for neonatal seizures, found that a normal or immature background strongly predicted the absence of electrographic seizures in subsequent 18- to 24-hour period of video-EEG monitoring, while background abnormalities predicted the occurrence of electrographic seizures within that same time frame (59). Among the 27 survivors of neonatal seizures followed for a mean of 31 months by Clancy and coworkers, postneonatal epilepsy occurred in 68% (13 of 19) of patients with moderately or markedly abnormal EEG backgrounds but in only 25% (2 of 8) without (60). Ortibus and colleagues however, did not find background abnormalities to be a strong predictor of seizures past the neonatal period (56).
Diagnosis and Treatment of Acute Seizures
Etiology
Major etiologies for acute seizures in the neonate include (i) neonatal encephalopathy and hypoxic-ischemic encephalopathy; (ii) intracranial hemorrhage and infarctions; (iii) infections; (iv) metabolic disturbances; (v) congenital structural brain lesions; and (vi) drug withdrawal (25,61). The term neonatal encephalopathy has gained favor in the literature over recent years over the more specific term, hypoxic-ischemic encephalopathy, as oftentimes, a direct causal relationship between hypoxia and encephalopathy is hard to establish.
Metabolic disturbances causing seizures are important to identify as many acute disturbances are potentially treatable with etiology-specific therapy. These include electrolyte abnormalities such as hypocalcemia, hypomagnesemia, and hypoglycemia.
Rare inborn errors of metabolism such as urea cycle defects, aminoacidurias, and organic acidurias can cause neonatal seizures refractory to AED treatment. These usually require dietary manipulation to control. Pyridoxine-dependent seizures can produce severe seizures, including spasms that may respond to pyridoxine.
Systemic and CNS infections can be secondary to postnatally acquired bacterial and viral agents, or congenital, in-utero infections.
Common acquired structural brain lesions include intracranial hemorrhage and cerebral infarctions. Focal areas of cerebral dysgenesis and more diffuse anomalies from major congenital brain malformations, such as in lissencephaly, can produce seizures as well in the neonatal period. The presence of a structural brain lesion can be suggested by focal seizures, electrographically arising from the same region, or from asymmetries in voltage or frequency of background activities. Neuroimaging is essential when such abnormalities are found on EEG.
Drug withdrawal or intoxication is another cause of transient seizures in the newborn. History of maternal drug use or of administration of medications during delivery is suggestive of the diagnosis. The affected neonate can appear depressed and exhibit symptoms such as jitteriness that can be confused with seizures.
When infectious, metabolic, and structural causes of seizures are excluded, neonatal encephalopathy secondary to hypoxic injury is often implicated, especially if accompanied by other systemic signs of anoxia such as persistently low Apgar scores, systemic acidosis, and multiorgan involvement. Grading schemes and criteria used to diagnose neonatal encephalopathy vary and it is difficult to assess the true proportion due to asphyxia (62,63). Perlman and colleagues looked at various clinical and laboratory markers within the first hour of life that identify newborn infants at high risk for seizures secondary to hypoxia ischemia. In their study of 96 infants, significant relationships with seizures were found with the following variables: 5-minute Apgar score of 5 or less, the need for intubation in the DR, umbilical cord arterial pH of 7.00 or less, and base deficit of-14 mEq/L (64). In 49 infants monitored with continuous EEG, Murray and colleagues found similar positive predictive value for seizures for low Apgar scores, systemic acidosis, and the need for intubation.
Surgery for congenital heart disease in the neonatal period is a procedure that carries increased risk for seizures. Electrographic seizures were found in 11% of 183 post-op infants monitored by video-EEG in a study by Clancy and colleagues (65) (Table 25.1).
Differential Diagnosis
The differential diagnosis of neonatal seizures includes physiologic and nonphysiologic events that may be abnormal or a
variation of normal neonatal behavior. Infants withdrawing from in-utero drug exposure or who have suffered anoxic or acute metabolic insults can have tremulous or jittery movements, as well as exaggerated clonus (66,67). Unlike seizures, these movements are very stimulus sensitive and can be suppressed by repo-sitioning or restraint. Physiologic myoclonus is a common sleep phenomenon mistaken for seizures. Some infants have a particularly exaggerated form of it that persists for the first few months but is benign. Because of its occurrence in sleep it is called benign neonatal sleep myoclonus (68). Infants with hyperekplexia, a condition caused by a mutation in genes encoding for inhibitory glycine receptor, also tend to have prominent myoclonus in sleep or upon arousal. In addition, they have excessive startle response triggered by minimal tactile or auditory stimulus, as well as episodes of generalized tonic stiffening (68, 69, 70, 71, 72 and 73). As mentioned earlier, subtle sudden or repetitive mouth or limb movements can raise the suspicion for seizures, but can be part of normal neonatal behavioral repertoire. Apnea or changes in autonomic functions are rarely epileptic in origin when they occur in the absence of other autonomic or motor manifestations (45).
variation of normal neonatal behavior. Infants withdrawing from in-utero drug exposure or who have suffered anoxic or acute metabolic insults can have tremulous or jittery movements, as well as exaggerated clonus (66,67). Unlike seizures, these movements are very stimulus sensitive and can be suppressed by repo-sitioning or restraint. Physiologic myoclonus is a common sleep phenomenon mistaken for seizures. Some infants have a particularly exaggerated form of it that persists for the first few months but is benign. Because of its occurrence in sleep it is called benign neonatal sleep myoclonus (68). Infants with hyperekplexia, a condition caused by a mutation in genes encoding for inhibitory glycine receptor, also tend to have prominent myoclonus in sleep or upon arousal. In addition, they have excessive startle response triggered by minimal tactile or auditory stimulus, as well as episodes of generalized tonic stiffening (68, 69, 70, 71, 72 and 73). As mentioned earlier, subtle sudden or repetitive mouth or limb movements can raise the suspicion for seizures, but can be part of normal neonatal behavioral repertoire. Apnea or changes in autonomic functions are rarely epileptic in origin when they occur in the absence of other autonomic or motor manifestations (45).
Table 25.1 Most Frequently Identified Etiologies of Neonatal Seizuresα | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree