Sensory Localization
Diminution or loss of sensation may occur because of lesions involving the peripheral nerves, nerve roots, spinal cord, brainstem, or higher centers of the brain, as may abnormal sensations, such as pain or paresthesia. Localization depends on the pattern and distribution of the sensory abnormality.
The primary modalities may be impaired because of disease involving peripheral nerve, spinal root, or sensory pathways within the central nervous system (CNS). When the primary modalities are normal in a particular body region, but the cortical modalities are impaired, a parietal lobe lesion may be responsible. When some primary modalities are involved more than others, the sensory loss is said to be “dissociated.” The pathways conveying pain and temperature (the spinothalamic tracts) run in a different location than the pathways conveying touch, pressure, position, and vibration (the posterior columns and medial lemniscus). After running divergently through much of their central course, the sensory pathways converge again as they approach the thalamus and remain together in the thalamocortical projections. When the pathways are close together, such as in the peripheral nerve, spinal root, or thalamus, disease processes tend to affect all primary modalities to an approximately equal degree. When the pathways are remote from each other, such as in the spinal cord and brainstem, a disease process may affect one type of sensation and not another, producing dissociated sensory loss. A common example of dissociated sensory loss is lateral medullary stroke, or Wallenberg syndrome. There is a very characteristic pattern of sensory loss, which only involves pain and temperature and completely spares light touch. The pain and temperature loss involves the ipsilateral face because of involvement of the spinal tract of cranial nerve V, and the contralateral body because of damage to the lateral spinothalamic tract, sparing the light touch pathways that are running in the midline in the medial lemniscus. A classic but not common cause of dissociated sensory loss is syringomyelia. The pain and temperature sensory fibers crossing in the anterior commissure are affected; light touch sensory fibers running in the posterior columns are well removed from the site of the pathology and remain intact. As a result, syringomyelia characteristically causes sensory loss to pain and temperature with preservation of light touch. Anterior spinal artery stroke is another example of dissociated sensory loss. The infarction involves the anterior two-thirds of the cord, sparing the posterior columns, which are perfused by the posterior spinal arteries. The patients have dense motor deficits and dense sensory loss to pain and temperature, but normal touch, pressure, position, and vibration. Patients with Brown-Séquard syndrome have extreme dissociation of modalities, with loss of pain and temperature on one side of the body and loss of touch, pressure, position, and vibration on the other side of the body.
In contrast, disease processes affecting a peripheral nerve trunk or a spinal root tend to involve all of the sensory fibers traveling in that nerve or root. The sensory loss involves all modalities. Occasionally, generalized polyneuropathies may have a predilection for large or small fibers, and can cause some differential involvement of pain and temperature as opposed to touch and pressure. These neuropathies are uncommon and tend to be generalized. When there is marked sensory dissociation affecting one body region, the pathology is virtually always going to be in the CNS, specifically in those regions where the different sensory pathways run in widely divergent locations.
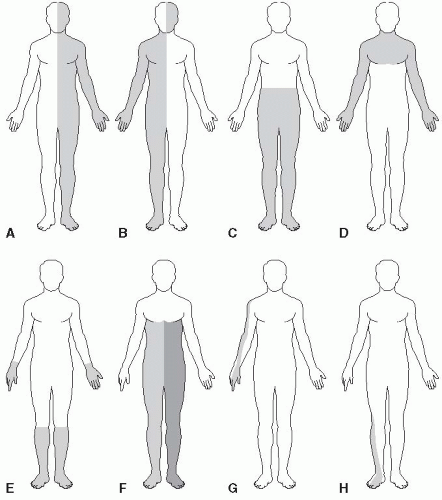
The other consideration in elucidating the cause of sensory loss, in addition to the modalities involved, is the distribution of the abnormality. Deficits in a “hemi” distribution obviously suggest CNS disease, likely involving either the cortex or the thalamus. Crossed deficits, affecting the face on one side and the body on the opposite side, suggest brainstem disease. Deficits involving both sides of the body below a certain level (e.g., T5) suggest spinal cord disease. A spinal cord level with “sacral sparing” suggests intraparenchymal spinal cord pathology rather than a myelopathy due to external pressure. Deficits due to generalized peripheral nerve disease typically involve the most distal body regions in a “stocking-glove” distribution. Sensory loss due to dysfunction of a peripheral nerve, nerve root, or nerve plexus follows the innervation pattern of that particular structure. Figure 26.1 depicts some of the commonly seen patterns of sensory loss. In hemi-distribution sensory loss there is a certain amount of side-to-side crossing or overlap of innervation along the anterior midline, which is greater on the trunk than on the face. Because of this midline overlap, organic sensory loss usually stops short of the
midline, while nonorganic sensory loss may “split the midline” (see further on). Sacral sensation is not tested as part of a routine neurologic examination. In some instances, sensation in the saddle distribution should be examined (e.g., when a conus medullaris or cauda equina lesion is a possibility; when there is evidence of a myelopathy; or when there is bladder, bowel, or sexual dysfunction).
midline, while nonorganic sensory loss may “split the midline” (see further on). Sacral sensation is not tested as part of a routine neurologic examination. In some instances, sensation in the saddle distribution should be examined (e.g., when a conus medullaris or cauda equina lesion is a possibility; when there is evidence of a myelopathy; or when there is bladder, bowel, or sexual dysfunction).
Sensory function and motor activity are interdependent, and severe motor disabilities may occur because of impaired sensation. This is particularly evident with parietal lobe lesions, but motor dysfunction may also occur with lesions involving the posterior roots, peripheral nerves, posterior columns of the spinal cord, or the other central sensory pathways. Conversely, motor dysfunction may affect sensory discrimination. When equal weights are placed in a patient’s hands, she may underestimate the weight on the side with cerebellar dysfunction and overestimate it on the side with extrapyramidal dysfunction.
Diminution or perversion of sensation may occur with pathology involving the sensory receptors, but this does not often arise in primary neurologic illnesses. Pain and pruritus due to skin irritation, traumatic denudements, and burns may result from abnormalities of the receptors or the nerve filaments to them, and decreased sensation in callosities and scars may result from involvement of the end-organs and smaller filaments.
In focal peripheral neuropathies, the area of sensory abnormality corresponds to the distribution of the specific involved nerve. The areas of skin supplied by various nerves are shown in Figure 26.2. Within the involved area, all sensory modalities are affected. Sensory distributions
may vary slightly from individual to individual, and the mapped area may not correspond precisely to a published text or atlas. An excellent source for a pictorial/graphic demonstration of peripheral nerve distributions is http://www.neuroguide.com/nerveindex.html.
may vary slightly from individual to individual, and the mapped area may not correspond precisely to a published text or atlas. An excellent source for a pictorial/graphic demonstration of peripheral nerve distributions is http://www.neuroguide.com/nerveindex.html.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree