CHAPTER 206 Skull Tumors and Fibrous Dysplasia
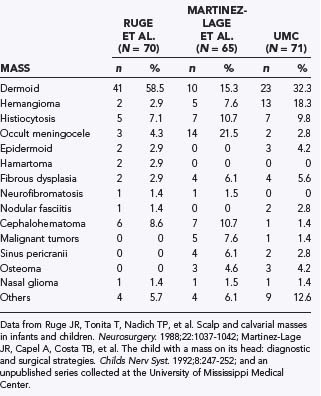
Because such disparate disciplines have been reporting the clinical series, epidemiologic studies have varied considerably in the incidence of the different types of lesions. Variations are noted in the clinical findings associated with the lesion, location, proximity to the skull base, multiplicity of lesions, and degree of intracranial involvement. Therefore, reports on the incidence and types of scalp and skull masses may differ not only because of the focus of a particular institution but also because of the specialty of the physicians collecting the series (Table 206-1).
Inclusion Cysts
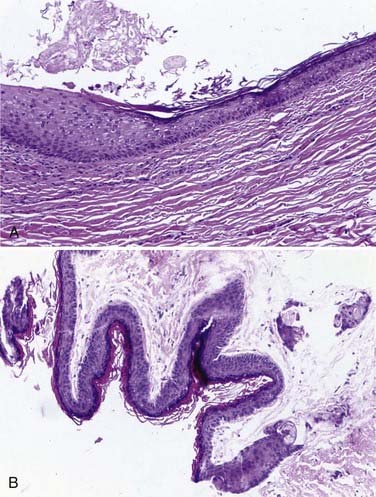
Approximately 50% to 60% of all scalp and skull lesions found in pediatrics are classified as either dermoids or epidermoids (see Table 206-1).1,2 These lesions involve the calvarial bone (especially the outer table), and in some cases the inner table is eroded and the outer leaf of the dura is involved. Dermoids occur more frequently, at a range of 15% to 58%, whereas epidermoids are noted in 3% to 5% of patients. Pathologic analysis differentiates strictly between the two lesions (Fig. 206-1). Epidermoids are composed of only keratin-producing epithelial cells and are characterized by cystic contents with white cheesy keratin debris. Dermoid cysts, in contrast, have walls that are more complex, including the cutaneous adnexal cells of the lower layers of the skin combined with sebaceous and sweat secretions produced by exocrine glands.
Although inclusion cysts may be found anywhere in the scalp, dermoid cysts develop more frequently along the midline in the area covered by the anterior and posterior fontanelles, and epidermoids tend to occur laterally at suture sites between the pterion and the asterion along the squamosal suture.3
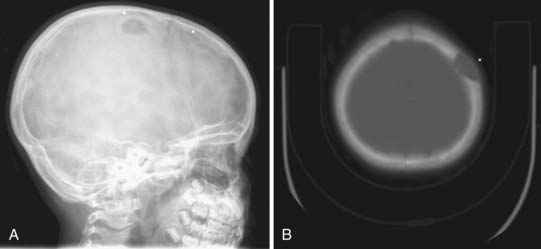
On plain radiographs, a lucent center is identifiable and accompanied by an eroding outer table or by involvement of the full thickness of the skull and a rim of hypersclerosis. Computed tomography (CT) will confirm that the lesion is extradural by displaying evidence of bone erosion. Magnetic resonance imaging (MRI) often shows the cyst to have a hypodense or hyperdense core, and the cyst wall may also enhance (Fig. 206-2).4,5
In managing these lesions surgically, a curvilinear incision around the lesion is preferable to an incision on the vertex of the lesion. A curvilinear incision minimizes the risk of incising into the cyst and thereby contaminating the surgical field. Ideally, the cyst should be excised without rupturing it. Although separating the cyst from the surrounding subcutaneous tissue and bone is usually quite simple, a small fibrous or vascular pedicle arising from the suture may require curettage or cauterization. These lesions can occasionally be manifested as a dumbbell configuration affixed to the underlying dura. If the bone defect is very large, reconstruction with split-thickness cranial bone may be necessary. Sharp and scalloped bony margins should be smoothed with a bur, drill, or curet. Incomplete removal of these lesions may lead to recurrence.6–8
Langerhans Cell Histiocytosis
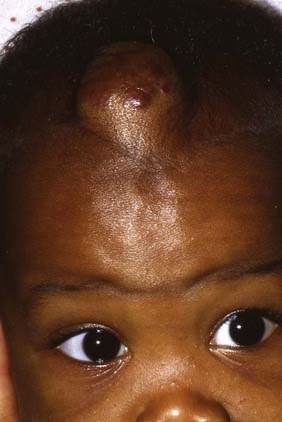
Historically, this entity has been referred to variously as histocytosis X or eosinophilic granuloma; Hand-Schüller-Christian disease and Letterer-Siwe disease are sometimes classified as systemic forms of histiocytosis related to Langerhans cell histiocytosis. Accounting for 7% to 10% of all reported skull lesions, this entity is a non-neoplastic lesion believed to be an inflammatory response to an unknown stimulus.9 It may be a solitary lesion classically referred to as an eosinophilic granuloma, or it may extend to multiple lesions, especially in children younger than 2 years. In its systemic manifestations, the process can disseminate into the central nervous system and through the viscera, thereby generating skin lesions, pulmonary infiltrates, hepatosplenomegaly, pancytopenia, and lymphadenopathy. An intermediate chronic form of this entity features lesions involving the calvaria in the granulomatous process, as well as exophthalmos and diabetes insipidus (obviously affecting the neurohypophysial structures). A recent study has associated degenerative central nervous system histiocytosis with this chronic process.10
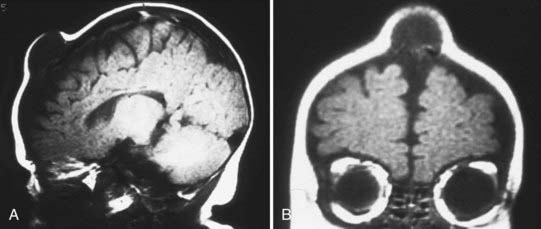
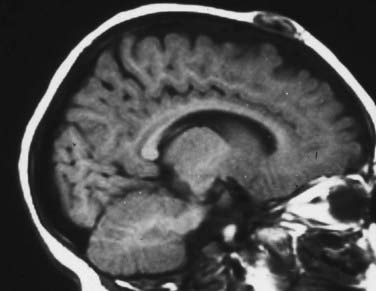
Skull radiographs show an osteolytic lesion with unequal involvement of the outer and inner tables, accompanied by a hyperostotic or sclerotic rim (Fig. 206-3A). CT demonstrates this expansile lesion to best advantage, especially with reconstructed bone formats. Even though the lesion usually enhances moderately, it can have a hemorrhagic core that is hyperdense on non–contrast-enhanced images. Although MRI will confirm the aforementioned findings in soft tissue, it defines the bone changes less clearly than CT does (Fig. 206-3B).
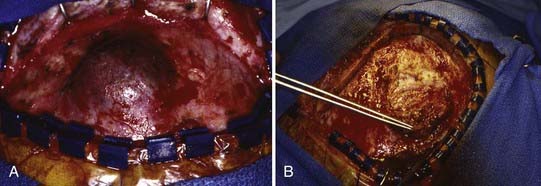
Management of these lesions involves wide surgical resection to normal bone margins (Fig. 206-4). The skull lesions can be attached to the outer leaf of the dura or to subcutaneous tissue, from which the lesion can usually be safely removed by curettage. These friable lesions are quite vascular and expansile within the outer and inner tables of the calvarial bone. Despite occasional location over the venous sinuses, vascular invasion is not seen in radiologic studies of these lesions. Persistence or recurrence of these lesions may require further treatment, including low-dose chemotherapy or, rarely, low-dose radiation therapy.
Hemangioma
Hemangiomas account for approximately 3% to 20% of reported skull and scalp lesions. They are classified into capillary and cavernous forms. An unusual osseous form that has been noted in adults is rarely seen in children. Hemangiomas tend to occur in the midline but may arise in the diploic spaces, where they extend outward and rarely involve the inner table of the skull, unless their blood supply originates from the dura.11
These lesions may initially be noted primarily as a simple cosmetic deformity or secondarily after trauma as a source of bleeding (Fig. 206-5). In some cases, compression, necrosis, and infection have been identified. In older children, hemangiomas have been accompanied by focal headaches, presumably caused by stretching of the surrounding pericranium.
Scalp hemangiomas are best imaged with MRI, which should confirm that the lesions are confined extradurally, especially when they are located in the area of the anterior fontanelle (Fig. 206-6). Either plain radiographs or CT scans provide the clearest images of the bony form of these lesions with sunburst-radiating trabeculae. The vertical orientation of this trabecular honeycombed arrangement of the skull is especially well visualized on tangential cross sections (Fig. 206-7).
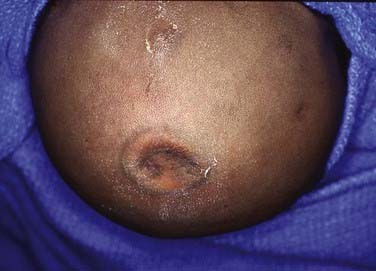
Occult Meningoceles
Cutis aplasia tends to be found over the cranial vertex along the sutural lines (Fig. 206-8). It may vary from less than 1 cm in size to very large scalp lesions (>10 cm). The resulting scalp defect may include the absence of bone formation and even a dural defect. Although smaller lesions (<3 cm) will spontaneously re-epithelialize, larger lesions (especially those with bone and dural defects) will require surgical reconstruction. Involvement of a plastic surgeon for skin rotation flaps, skin grafts, or tissue expanders is highly recommended for these larger lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree