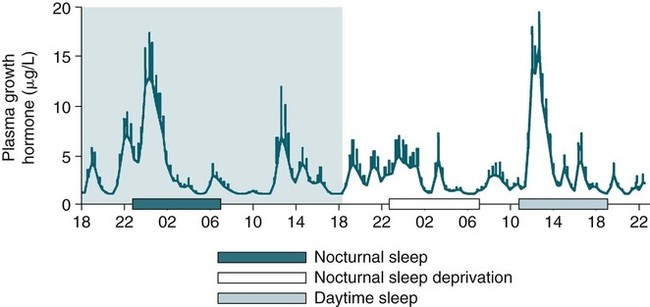
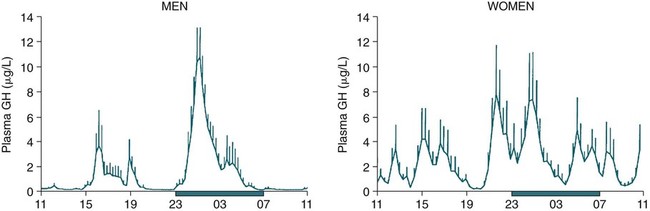
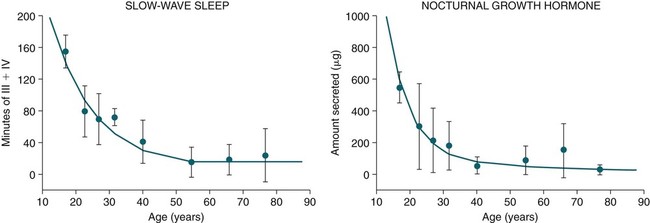
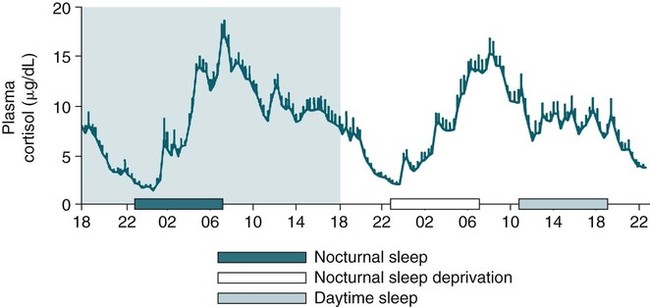
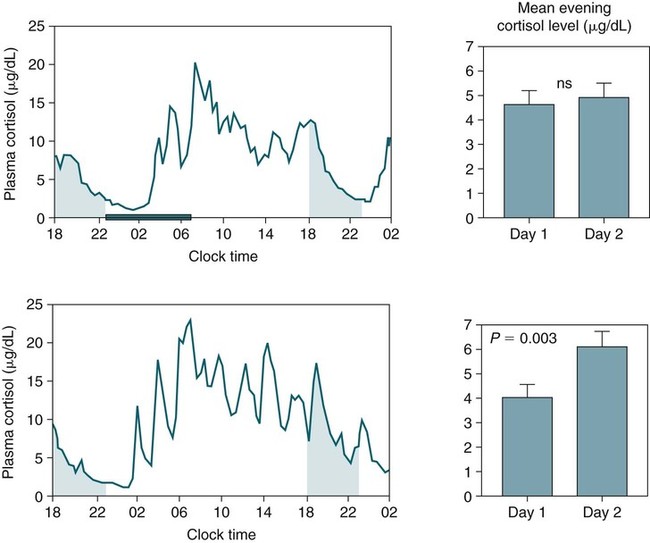
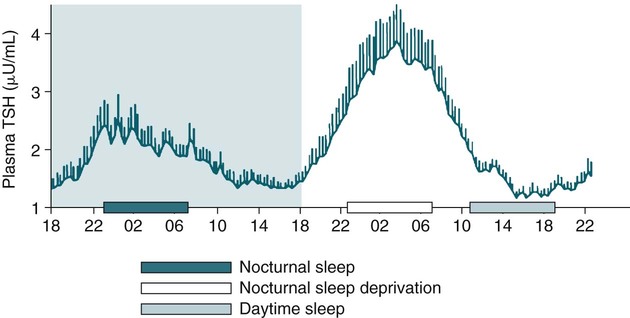
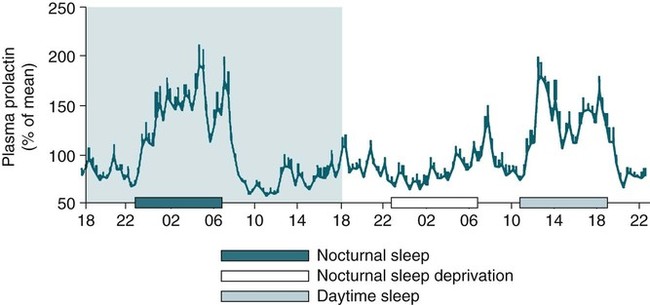
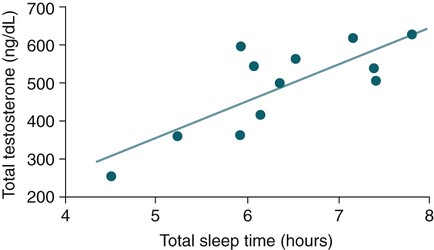
• The secretion of GH and PRL by the pituitary is controlled mainly by the timing of sleep. GH secretion in men is tightly tied to the first cycle of stage N3 sleep. PRL secretion is increased during sleep (inhibited by wakefulness). • The secretion of ACTH, cortisol, and TSH are mainly controlled by circadian timing (time of day) influences with weaker sleep-related effects. The secretion of ACTH and cortisol peaks soon after awakening. TSH secretion is under circadian control (peaks during the night). Sleep inhibits TSH secretion. • Ghrelin is a hormone secreted by the stomach that stimulates the appetite and is increased by sleep loss. Leptin is a hormone secreted by adipose tissue that increases satiety. Leptin is decreased by sleep loss. Studies suggest that individuals with decreased total sleep time have an increased risk of developing obesity. • The normal defense mechanisms to minimize the detrimental effects of GER are not present during sleep. Saliva secretion virtually stops and reflex swallowing to clear refluxed material is not present. This results in a prolonged ACT. The absence of symptoms does not eliminate the presence of GER. Esophageal pH monitoring often shows significant GER episodes in asymptomatic patients. • Nocturnal GER is common in patients with OSA and is improved with CPAP treatment. • Alpha-delta sleep (alpha anomaly) is not specific to fibromyalgia. Alpha anomaly may be seen in psychiatric disorders, in many chronic pain syndromes, and in normal individuals. • Sleep in patients with the FS is abnormal and studies have shown decreased total sleep time, increased arousals, and decreased stage N3 and REM sleep. At least in some patients, a poor night of sleep is associated with worse daytime symptoms. Growth hormone (GH) is secreted by somatotroph cells of the anterior pituitary under hypothalamic control. GH secretion is increased by GHRH (growth hormone–releasing hormone) and decreased by somatostatin, both secreted by the hypothalamus. GH secretion is also increased by acylated ghrelin (ghrelin is secreted by the stomach). The most reliable burst of GH secretion is associated with the first slow wave sleep (stage N3) cycle (any time of the day).1,2 This is associated with increased GHRH and decreased somatostatin. In healthy adults, the 24-hour profile of plasma GH consists of stable low levels abruptly interrupted by bursts of secretion (Box 30–1 and Fig. 30–1). The most reproducible GH pulse occurs shortly after sleep onset. In men, the sleep-onset GH pulse is the largest and often the only secretory pulse over the 24-hour day. In women, daytime GH pulses are more frequent and the sleep-onset pulse does not account for the majority of the 24-hour secretory output (Fig. 30–2). The amount of GH released is proportional to slow wave activity. Figure 30–1 shows a large burst of GH secretion after sleep onset. During the next nighttime period, the subject is awake and there is only a slight increase in GH secretion. The subject is allowed to sleep at 11 am, and sleep onset is followed by a large burst in GH secretion. Injections of GHRH stimulate stage N3 and slow wave activity (electroencephalogram [EEG] spectral power in the delta range). In contrast, injections of GH appear to enhance rapid eye movement (REM) sleep, particularly in rodents.3 Somatostatin injections impair sleep quality in older, but not in young, adult humans.4 The nocturnal increase in GH decreases with age in parallel with the amount of stage N3 (formerly termed stages 3 + 4; Fig. 30–3).5 The adrenals secrete cortisol under the control of pituitary adrenocorticotropic hormone (ACTH). There is strong circadian control of ACTH secretion (and cortisol). The plasma levels of cortisol and ACTH peak in the early morning and decline during the day (Box 30–2 and Fig. 30–4). The nadir of cortisol and ACTH levels is during the first part of sleep. The levels start to climb a few hours before waking. During shifted sleep, the cortisol rhythm remains tied to clock time. There is a weak sleep-related component. The presence of sleep causes inhibition and maintains low levels early in the sleep period (Fig. 30–5). Awakening at the end of sleep is associated with a burst of cortisol secretion. Sleep deprivation induces a 15% decrease in amplitude of the 24-hour cortisol rhythm (i.e., peak to trough of excursion is smaller). In the absence of sleep, the nadir in the early part of the sleep period is not as low and the peak following in the morning is not as high (absence of stimulating effects of awakening). However, sleep fragmentation causes a burst of cortisol and higher morning levels. The night of sleep deprivation induces less recovery (less impact on morning stimulation by ACTH). Older adults tend to have higher evening cortisol levels (Fig. 30–6; see also Box 30–2). Daytime levels of plasma TSH are low and relatively stable. There is a rapid elevation of TSH starting in the early evening and culminating in a nocturnal maximum occurring around the end of the first third of the sleep period (Box 30–3 and Fig. 30–7). The latter part of the sleep period is associated with a progressive decline in TSH. Because the start of the nocturnal rise in TSH occurs well before sleep onset, it likely reflects a circadian effect. Sleep exerts an inhibitory influence on TSH secretion,1,2 and sleep deprivation relieves this inhibition, resulting in higher TSH during the night. The inhibition of TSH by sleep is greatest during stage N3 sleep and the inhibition is greater on recovery sleep from prior sleep deprivation (more stage N3, higher delta power). PRL levels show a bimodal pattern. They are minimal around noon, increase somewhat during the afternoon, and then increase shortly before sleep onset (Fig. 30–8). Decreased dopaminergic inhibition of PRL during sleep is likely to be the primary mechanism underlying nocturnal PRL elevation. In adults of both sexes, the nocturnal maximum corresponds to an average increase of more than 200% above the minimum level. Morning awakenings and awakenings interrupting sleep are consistently associated with a rapid inhibition of PRL secretion. Studies of the PRL profiles during daytime naps or after shifts of the sleep period have consistently demonstrated that sleep onset, irrespective of the time of day, has a stimulatory effect on PRL release1,2 (Box 30–4). Note that the stimulatory effect of sleep on PRL secretion is greatest at night. There is also some evidence for an involvement of PRL in stage N3 regulation. Stage N3 sleep is enhanced in patients with hyperprolactinemia and in women who breast-feed and have high PRL levels compared with women who bottle-feed their infants.6,7 A summary of the control of secretion of GH, cortisol, TSH, and PRL is listed in Table 30–1. The table highlights that the secretion of some hormones is tied to sleep and others to circadian control. There may be a weaker secondary effect of sleep on hormone secretion primarily under circadian control and a weaker circadian effect on hormone secretion primarily under sleep control. A mnemonic for remembering the major control mechanisms: GPS – ATC = Growth hormone and Prolactin under Sleep-wake control and ACTH (cortisol) and TSH primarily under Circadian control. TABLE 30–1 Summary of Major Control of Major Hormones ACTH = adrenocorticotropic hormone; GH = growth hormone; TSH—thyroid-stimulating hormone. The 24-hour patterns of gonadotropin release and gonadal steroid levels vary according to the stage of life and are gender dependent.8,9 In prepubertal children, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are secreted in pulses of low amplitude, and a sleep onset–augmentation effect is present in the majority of both girls and boys. As the child approaches puberty, the amplitude of the nocturnal pulses increases and the diurnal rhythm becomes more evident. A large nocturnal increase in gonadotropins is one of the hallmarks of puberty. In pubertal boys, the nocturnal rise of testosterone parallels the elevation of gonadotropins, whereas in pubertal girls, higher concentrations of estradiol occur during the daytime instead of the nighttime.9 It has been proposed that the lack of parallelism between the diurnal variation of gonadotropins and estradiol reflects a 6- to 10-hour delay between gonadotropin stimulation and the ovarian response related to the time required for aromatization of estradiol. In young adult men, the day-night variation of plasma LH levels is dampened or even undetectable,10 whereas a marked diurnal rhythm in circulating testosterone levels persists with minimal levels in the late evening, and a clear nocturnal elevation results in maximal levels in the early morning.11 Sleep fragmentation in normal men decreases the nocturnal rise in testosterone if REM sleep does not occur. The sleep related rise in testosterone appears to be linked to the first REM period.11,12 In elderly men, the morning level of testosterone depends on the amount of sleep. Higher levels correlate with more sleep. A recent study has indicated that the amount of nighttime sleep is a strong predictor of morning testosterone levels in healthy older men13 (Fig. 30–9). Appetite is regulated by the interaction between metabolic and hormonal signals and neural mechanisms.14 The arcuate nucleus of the hypothalamus has two opposing sets of neuronal circuitry, appetite-simulating and appetite-inhibiting, and several peripheral hormonal signals have been identified that affect these neuronal regions.15 The peripheral signals included leptin and ghrelin (Box 30–5). Leptin is primarily secreted by adipose tissue and appears to promote satiety.15 Ghrelin is a peptide released primarily from the stomach.16 Studies in humans also indicate that ghrelin increases appetite and food intake. Plasma ghrelin levels are rapidly suppressed by food intake and then rebound after 1.5 to 2 hours, paralleling the resurgence in hunger. Thus, leptin and ghrelin exert opposing effects on appetite. Animal studies suggest that leptin and ghrelin also have opposing effects on energy expenditure (leptin increasing energy expenditure), but the picture is less clear in humans. Under normal conditions, the 24-hour profile of human plasma leptin levels shows a marked nocturnal rise. When leptin levels were studied with continuous enteral nutrition, leptin levels still rose at night.17 Ghrelin levels typically rise during the first half of the night, then decrease in the second half even in the fasting condition.18 A mnemonic for the effect of Ghrelin is “Gotta Have Food.”
Sleep and Nonrespiratory Physiology—Impact on Selected Medical Disorders
Growth Hormone
GHRH and GH—Effects on Sleep
Changes in GH Secretion with Age
Sleep and the Corticotropic Axis
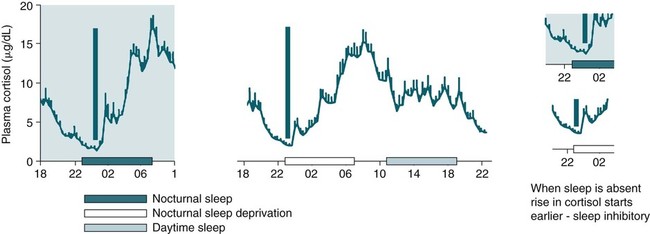
Sleep Deprivation and Sleep Loss
Sleep and the Thyroid Axis
TSH Secretion
Sleep and Prolactin Secretion
Evidence for the Role of PRL in Regulation of Sleep
Summary of Control of Hormone Secretion
HORMONE
PRIMARY
SECONDARY
GH
Sleep (stimulatory, onset stage N3)
Weak circadian effect (increased GH secretion at night)—low somatostatin?
ACTH, cortisol
Circadian (peaks around 7 am)
Sleep inhibitory
TSH
Circadian (peaks around 2 am)
Sleep inhibitory
Prolactin
Sleep (stimulatory)
Circadian effect—sleep is more stimulatory when it occurs at night
Sleep and the Gonadal Axis
Leptin and Ghrelin
Sleep and Nonrespiratory Physiology—Impact on Selected Medical Disorders








