A specific type of CBT-I is stimulus–control therapy, which is useful in the management of conditioned insomnia. This technique is an attempt to break the conditioning by teaching the patient to associate the bedroom with sleep behavior. The instructions for sleep hygiene and stimulus–control behavioral therapy are listed in Tables 60.1 and 60.2. Sleep restriction therapy involves curtailment of time in bed, so that sleep efficiency (time asleep divided by time in bed) is 85% or greater. As sleep efficiency increases, time in bed is gradually lengthened. Shorter-duration behavioral interventions, and for patients who do not have access to formal face-to-face CBT-I training, Internet-based approaches are also available.
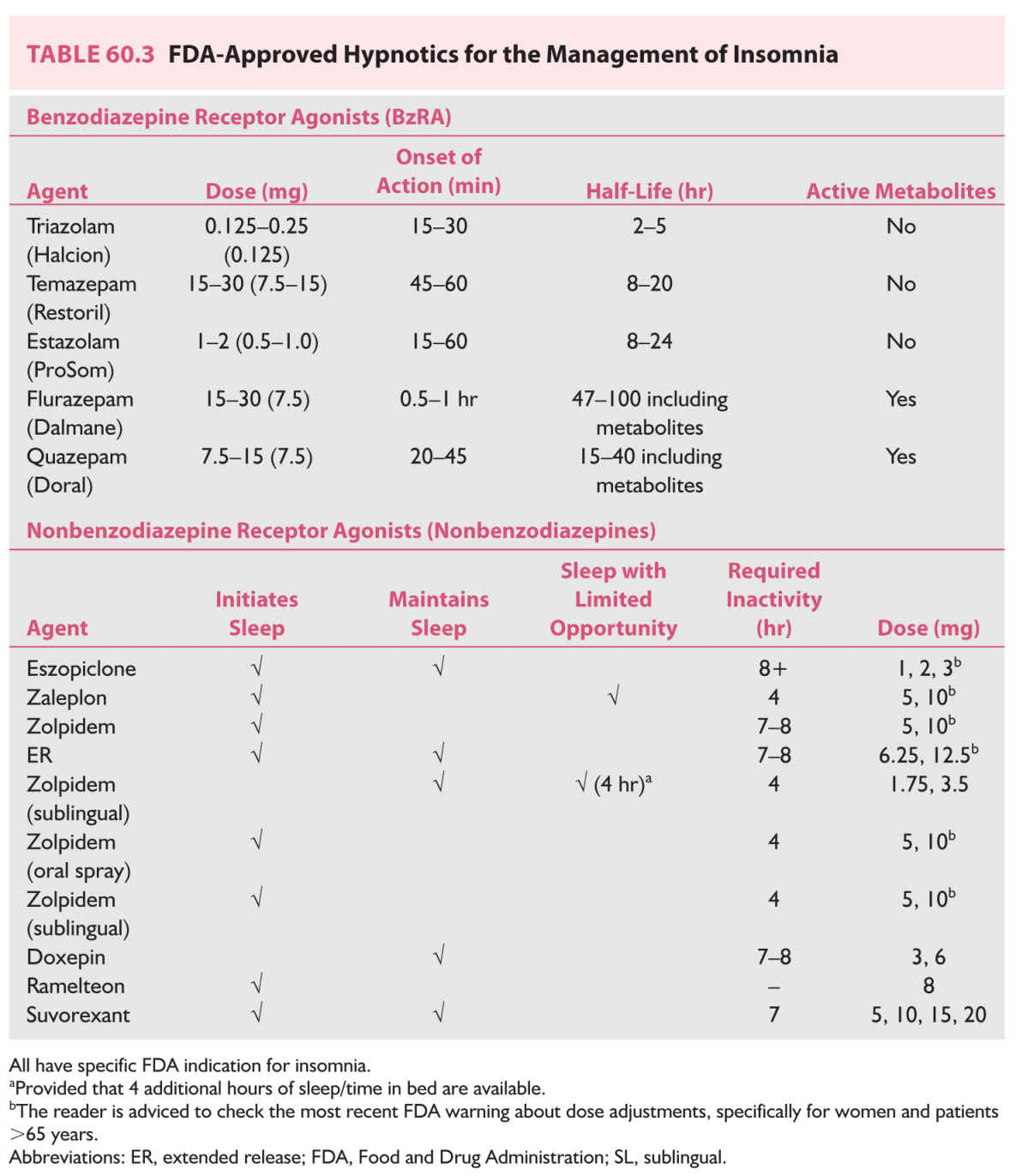
2. Hypnotic drugs. The most widely used prescription hypnotics are the benzodiazepine receptor agonists, which include benzodiazepines (BzRA) and the nonbenzodiazepine receptor agonist (non-BxRA) hypnotics, such as eszopiclone, zaleplon, and zolpidem. Traditionally, this class of medications was indicated for short-term use. More recently, with the recognition that insomnia is often chronic and with the availability of longer-term studies for up to a year, the short-term indication has been removed from the newly Food and Drug Administration (FDA)-approved hypnotics such as eszopiclone and zolpidem extended release (ER). Ramelteon, a melatonin receptor agonist; low-dose doxepin; and suvorexant, an orexin receptor antagonist, represent different classes of hypnotics with unique mechanisms of action that target the sleep/wake system.
The choice of hypnotic may depend on the timing of insomnia. For example, if the predominant difficulty is with sleep initiation, a fast-acting, short-half-life hypnotic may be preferable. If the problem is frequent awakenings during the night and sleep maintenance insomnia, a longer-acting hypnotic may be more effective. Most hypnotics approved by the FDA are indicated for the treatment of sleep-onset insomnia, whereas eszopiclone, zolpidem ER, low-dose doxepin, and suvorexant are also indicated for the treatment of sleep maintenance insomnia. In practice, sedating antidepressants, such as the tricyclic antidepressants and heterocyclics (trazodone), are frequently used off label for the treatment of insomnia. However, there is limited data regarding their efficacy or long-term safety for the treatment of insomnia that is not comorbid with depression. The exception is low-dose doxepin (3 to 6 mg), which is FDA approved for insomnia.
The most widely used prescription hypnotics and their properties are listed in Table 60.3. Although patients with chronic insomnia rarely become “great” sleepers after treatment, most can manage the perpetuating factors of insomnia by using sleep hygiene, cognitive-behavioral treatment, and, when indicated, hypnotics.
INSOMNIA ASSOCIATED WITH NEUROLOGIC, PSYCHIATRIC, AND MEDICAL DISORDERS
A. Course. Insomnia is often comorbid with neurologic and psychiatric conditions. Results of epidemiologic studies suggest that as many as 57% of persons with insomnia have a comorbid psychiatric condition or will have one within 1 year. The comorbid condition is usually a mood disorder, anxiety disorder, somatoform disorder, personality disorder, schizophrenia, pain syndrome, or substance abuse. Sleep in major depression is usually characterized by early morning awakening (2 to 4 hours after sleep onset), and frequent nocturnal awakening with inability to reinitiate sleep. Although depression and insomnia have a bidirectional relationship, insomnia often precedes the diagnosis of depression. The incidence of insomnia among patients with anxiety disorders is high. The typical symptoms are difficulty with sleep initiation and, to a lesser degree, nocturnal awakenings. Fatigue is common, but napping is unusual.
B. Treatment and outcome. Treatment should address the comorbid medical, neurologic, and psychiatric disorders as well as insomnia. For major depressive and anxiety disorders, this involves use of antidepressants or anxiolytics such as the selective serotonin reuptake inhibitors (SSRIs). An antidepressant with sedative properties is favored over a less-sedating one for patients with insomnia. Administration of the drug 30 minutes before bedtime also aids in promoting sleep.
Amitriptyline, trimipramine, doxepin, trazodone, and mirtazapine are the most sedating, whereas protriptyline and SSRIs such as fluoxetine and serotonin-norepinephrine reuptake inhibitors (SNRIs) have stimulating effects that may worsen insomnia and are best to be prescribed in the early part of the day. Antidepressants with anxiolytic properties are useful in the treatment of anxious, depressed patients and facilitate psychotherapeutic or pharmacologic treatment. Anticholinergic side effects of tricyclic antidepressants (cardiotoxicity, urinary retention, erectile dysfunction, and dry mouth) limit the usefulness of these agents, particularly in older patients.
Recent studies demonstrate that insomnia may persist despite adequate treatment of depression and that insomnia predicts future relapse of depression. Therefore, oftentimes, a parallel approach that combines treatment for both depression and insomnia is recommended. If the patient is refractory to treatment, a referral to a sleep specialist or psychiatrist is recommended for further evaluation of comorbid psychiatric or other underlying comorbid sleep disorders.
CIRCADIAN RHYTHM SLEEP DISORDERS
Circadian rhythms are generated by the internal neural clock located in the suprachiasmatic nucleus of the hypothalamus. Disruption of biologic timing, or the alignment between internal circadian rhythms with the external physical or social environment, results in circadian rhythm disorders that are most often associated with patients’ reports of difficulty initiating and maintaining sleep, and excessive sleepiness. However, their impact extends beyond insomnia or hypersomnia to adverse health outcomes, impairments in social, occupational, and educational performance, and safety concerns.
CRSWDs are characterized by essentially normal total sleep time for age that is not synchronized with conventional environmental light–dark cycles and periods of sleep. Diagnosis requires specialized assessment, including use of a sleep diary for at least 7 days alone, or in combination with actigraphy when possible, physiologic markers of circadian timing such as core body temperature or melatonin onset. A careful history interview to elicit the appropriate major diagnostic criteria is key. CRSWDs include delayed sleep–wake phase disorder (DSWPD), advanced sleep–wake phase disorder (ASWPD), non-24-hour sleep–wake disorder (N24SWD), irregular sleep–wake rhythm disorder (ISWRD), shift work sleep–wake disorder, and jet lag disorder.
Effective treatment of CRSWDs typically requires a multifaceted approach to realign circadian rhythms with the use of timed bright light exposure and low-dose melatonin, together with cognitive-behavioral treatments that promote healthy sleep habits. The reader should be reminded that melatonin is not approved by the FDA for the treatment CRSDs, and one should also be aware of potential side effects such as headaches, vivid dreams, nausea, and cardiovascular effects.
A. DSWPD and ASWPD.
1. Clinical course.
DSWPD is characterized by a persistent inability to fall asleep until the early morning hours (typically between 1 and 3 a.m., and sometimes later) and profound difficulty waking up in the morning. If allowed, the patient would sleep until the late morning or early afternoon (10 a.m. to 2 p.m.). When the patient is forced to rise at 7 or 8 a.m., sleep is curtailed, and daytime sleepiness develops. Despite the daytime sleepiness, patients find that in the evening they become more alert and remain unable to fall asleep until the early morning hours. The prevalence rate is estimated to be between 1.7% (in the general population) and 7% (of those with insomnia complaints). Onset of this disorder typically occurs during adolescence or early adulthood.
ASWPD is characterized by early evening sleep onset, (typically at 7 to 9 p.m.), and early morning awakening (between 3 a.m. and 5 a.m.). Although DSWPD predominates at younger ages and ASWPD at older ages, both disorders can result in sleep problems throughout the life span. Because many features of the sleep of patients with depression resemble those of DSWPD or ASWPD, depression and other psychiatric disorders must be considered in the differential diagnosis.
2. Treatment and outcome.
a. Chronotherapy is a behavioral technique in which bedtime is systematically delayed (for DSWPD) or advanced (for ASWPD) in 2- to 3-hour increments each day until the desired sleep phase is achieved. The patient is then instructed to maintain the newly established bedtime rigidly. Although this approach works, it is an arduous procedure, and maintenance of the effect has been difficult.
b. Bright light therapy. Light intensity greater than 1,000 lux is considered bright. Appropriately timed bright light (white or blue/green enriched) exposure can reset the timing of circadian rhythms, and normalize circadian phase in DSWPD and ASWPD. Exposure to bright light in the early morning results in an advancement of circadian phase, whereas exposure to light in the evening delays circadian rhythms. For management of DSWPD, exposure to light is usually scheduled for 1 to 2 hours in the morning (close to the time of habitual awakening). For ASWPD, light exposure is recommended in the evening, approximately 2 to 4 hours before scheduled bedtime. Avoidance of bright light in the evening in DSWPD should also be encouraged. Despite high rates of success in achieving the desired sleep phase under immediate treatment, many patients do not continue the light regimen and have a relapse. Some patients are able to maintain a normalized phase without maintenance of light exposure for as long as several months, whereas others drift back toward the pretreatment phase within a few days.
c. Melatonin has been shown to shift the phase of circadian rhythms in humans. Although not approved by the FDA, melatonin of 1 to 5 mg has been shown to be effective when taken in the early evening (5 to 6 hours before habitual falling asleep time) for patients with DSWPD.
B. N24SWD.
1. Clinical course. Individuals with N24SWD typically have a longer than 24-hour circadian rhythm, similar to those living in temporal isolation. Because these patients are unable to achieve stable entrain to the external 24-hour physical, social, or activity cycles, sleep and wake periods progressively drift later each day. Diagnosis of N24SWD includes complaints of insomnia or excessive sleepiness associated with the misalignment between the endogenous circadian rhythm and the light–dark cycle. N24SWD is most common in blind people, but can occur in sighted persons. There is an overlap between severe DSWPD and N24SWD.
2. Treatment and outcomes. Both behavioral and pharmacologic options are available for the treatment of non-24-hour sleep–wake rhythm disorder, depending on whether the patient is sighted or blind. For blind and sighted patients, planned sleep schedules and/or low-dose melatonin (0.5 to 3 mg) approximately 1 to 2 hours before habitual bedtime are recommended. Tasimelteon (20 mg), a melatonin receptor agonist, was recently approved by the FDA for the treatment of N24SWD. In sighted persons, the addition of timed exposure to bright light in the morning is also recommended.
C. ISWRD.
1. Clinical course. ISWRD differs from the phase disorders in that there is a low amplitude to a complete loss of circadian rhythmicity, which results in the lack of a long, consolidated sleep period. Sleep is usually broken into three or more short sleep periods or naps during the course of 24 hours. Irregular sleep–wake patterns occur among patients with neurodevelopmental and neurodegenerative disorders, particularly in Alzheimer’s disease and among other elderly persons in nursing homes.
2. Treatment and outcomes. Management of irregular sleep–wake patterns and associated behavioral problems in this group of elderly and often cognitively impaired patients is a challenge. Treatment with sedative-hypnotics is prevalent in nursing homes. These medications have side effects that may not be well tolerated by older patients. The most promising is a multicomponent approach with structured physical and social activities and increasing light exposure during the day and optimizing the sleep environment by reducing noise and light at night.
Studies have indicated that structured activity programs, increasing exposure to bright light and evening melatonin, may alleviate these sleep–wake and behavioral disorders. The effects of melatonin have been mixed, and a recent placebo-controlled multicenter study in Alzheimer’s disease failed to demonstrate its effectiveness. Light therapy units are commercially available.
D. Shift work disorder (SWD).
1. Clinical course. SWD is characterized by chronic symptoms of insomnia and excessive sleepiness that are due to unconventional work schedules, resulting in circadian misalignment. Typically, sleep is curtailed by 1 to 4 hours in patients with SWD, with most complaints associated with night and early morning work. Excessive sleepiness at work and commute poses important safety concerns.
2. Treatment and outcomes. Clinical management of SWD is aimed at realigning circadian rhythms with the sleep and work schedules, as well as improving sleep, alertness, and safety. Nonpharmacologic treatments are basic to the management of SWD. Optimizing the sleep environment, adherence to healthy sleep habits, and planned naps, when possible, should be encouraged for all patients.
a. Bright light therapy. Timed bright light therapy and avoidance of light at the wrong time of the day can help accelerate and maintain entrainment to the shift schedule. For night workers, circadian rhythms need to be delayed, so that the highest sleep propensity occurs during the day, rather than at night. Intermittent bright light exposure (~20 minutes/hour blocks) and avoidance of bright light exposure in the morning during the commute to home (using driving safe sunglasses) have been shown to accelerate circadian adaptation to night shift.
b. Melatonin. Studies on the effectiveness of melatonin for the treatment of SWD have been mixed. When taken at bedtime after the night shift, melatonin can improve daytime sleep, but may have limited effects on alertness at work. Other pharmaceuticals often used for the treatment of sleep disturbance and excessive sleepiness in shift workers include short-acting hypnotics for insomnia and FDA-approved stimulants such as modafinil and armodafinil for maintaining alertness. The required dose of these medications can vary among individuals and can be titrated. However, these approaches do not specifically address the issue of circadian misalignment, and thus should be used in concert with behavioral strategies as discussed above.
DISORDERS OF EXCESSIVE DAYTIME SLEEPINESS
Sleepiness severe enough to affect activities of daily living is estimated to be present among 30% of the population and is most commonly caused by self-imposed restriction of sleep. However, approximately 4% to 5% of the population has EDS as a result of a sleep disorder. Sleepiness is excessive and an indication of a sleep disorder when it occurs at undesirable times, such as while driving and during social activities. EDS can be divided into two types: extrinsic and intrinsic. Some extrinsic causes include environmental factors, drug dependency, sleep-disordered breathing, and movement disorders during sleep. The more common types of intrinsic hypersomnia usually associated with primary central nervous system (CNS) includes disorders such as narcolepsy and idiopathic hypersomnia (IH).
A. Narcolepsy.
1. Clinical features. Narcolepsy is a syndrome characterized by a pentad of (i) severe unremitting excessive sleepiness (occurs in 100% of patients with narcolepsy), and is the sinequanone of the disorder, manifesting as sleep attacks; (ii) cataplexy (found in 60% to 70% of patients); (iii) sleep paralysis (SP) (impacting 25% to 50% of patients); (iv) hypnagogic hallucination and/or hypnopompic hallucination (HH) (in 20% to 40% of patients); and (v) disturbed nocturnal sleep (in 70% to 80% of patients). A description of these symptoms is provided in Table 60.4. Some patients will also have automatic behavior (20% to 40%), other comorbid primary sleep disorders such as restless leg syndrome, periodic leg movement disorder, rapid eye movement (REM)–sleep behavior disorder, and REM sleep without atonia (RSWA) (see Section H 1 b iii). All patients must have pathologic levels of daytime sleepiness. Cataplexy is defined by a sudden loss of skeletal muscle tone induced by strong emotional stimuli, typically laughter or joking, and is a pathognomonic feature for narcolepsy. Cataplexy is associated with a drop in H-reflex.
Sleep apnea is noted in one-third of narcoleptic patients and may be related to the high body mass index (BMI) seen in narcolepsy patients, which could aggravate sleep attacks and is critical to screen for it, especially in patients whose sleepiness does not resolve with therapy or in overweight patients who snore.
2. Clinical course. Narcolepsy is a manifestation of dissociation between wakefulness and sleep, particularly REM sleep. It is characterized by inability to maintain wakefulness during the day, but also by an inability to maintain sleep during the night. The onset usually occurs in adolescence or young adulthood, and men are affected more often than women. The prevalence of narcolepsy in the United States is estimated to be approximately 1 in 2,000 individuals, with most cases being sporadic. Studies have shown a strong genetic association between narcolepsy and the human leukocyte antigen (HLA) type DR2 and DQ1. A more sensitive marker for narcolepsy is the HLA DQB1*0602 genotype, which appears to be correlated with both the frequency and severity of cataplexy.
The role of orexin [also known as hypocretin (Hcrt)] in narcolepsy is supported by the finding that Hcrt levels are abnormally low or undetectable in the cerebrospinal fluid of most narcoleptic patients. Values below 110 pg/mL are highly diagnostic for narcolepsy in the absence of severe brain pathology. The most consistent abnormalities were observed in the amygdala, where increased dopamine and metabolite levels were found.
The most plausible theory for the pathogenesis of narcolepsy–cataplexy syndrome suggests that the condition results from a depletion, either through degeneration or autoimmune disorder, of the Hcrt neurons, in patients who are at risk conferred by HLA-DQB1*0602 haplotype. New data reveal evidence of gliosis in the lateral hypothalamus, the location of the Hcrt neurons, prompting exploration for possible immune-related dysfunction in narcolepsy. Environmental triggers such as streptococcal infection and seasonal influenza, and more recent reports implicating the 2009 influenza pandemic A/H1N1 favor an immunologic mechanism for narcolepsy implicating a small epitope of H1N1, which resembles Hcrt and may be involved in molecular mimicry.
B. Classification of narcolepsy.
1. Narcolepsy type 1 (NT-1) or Hcrt (orexin) deficiency syndrome. (previously known as narcolepsy–cataplexy syndrome). Characterized by EDS for 3 months and at least one of the following:
a. Cataplexy, and on Multiple Sleep Latency Test, a Mean Sleep Latency of <8 minutes and >2 sleep-onset rapid eye movement sleep periods (SOREMPs). One SOREMP may be on the preceding night’s polysomnogram, OR
b. CSF Hcrt-1 levels <110 pg/mL or one-third the baseline normal levels, and on multiple sleep latency testing (MSLT), MSL < 8 minutes > 2 SOREMPs (one SOREMP may be on the preceding night’s polysomnogram)
2. Narcolepsy type 2 (NT-2, with normal Hcrt levels) (previously known as narcolepsy without cataplexy):
Unlike NT-1, there is absence of cataplexy and Hcrt levels are normal. Positive polysomnography/multiple sleep latency test is met [(mean sleep latency of <8 minutes and ≥2 SOREMPs on the MSLT. A SOREMP (within 15 minutes of sleep onset) on the preceding nocturnal polysomnogram may replace one of the SOREMPs on the MSLT].
3. Secondary narcolepsy. Because of a known underlying CNS disorder, it is referred to as narcolepsy type 1 because of a medical condition (when Hcrt levels are low) or narcolepsy type 2 because of a medical condition (when Hcrt levels are normal).
This condition is found in a number of key medical and neurologic conditions including abnormalities, genetic disorders associated such as Prader–Willi syndrome, structural lesions in the hypothalamic region, and inflammatory lesions such as multiple sclerosis and acute disseminated encephalomyelitis, midbrain tumors, vascular malformations, encephalitis, cerebral trauma, and paraneoplastic syndrome with anti-Ma2 antibodies and Niemann–Pick disease type C.
4. Diagnosis. In addition to the clinical history, nocturnal polysomnography (PSG) and MSLT are performed to establish a diagnosis of narcolepsy.
a. Sleep studies.
(1) PSG. A baseline sleep study is generally required for an accurate diagnosis of narcolepsy because of the spectrum of conditions that can cause excessive sleepiness. Most typically, the nocturnal PSG is required, followed by the MSLT. PSG features of narcolepsy include sleep disruption, repetitive awakenings, and decreased REM sleep latency. A SOREMP at night is highly predictive of narcolepsy and may be diagnostic of the condition along with the MSLT.
(2) The MSLT. The MSLT during the day following the PSG is designed to determine a patient’s propensity to fall asleep. As noted previously, the current criteria for narcolepsy include an MSL of ≤8 minutes and ≥2 SOREMPs, one of which may be a SOREM ≤15 minutes on the diagnostic polysomnogram.
However, up to one-third of the general population may have an MSL of ≤8 minutes, so the finding of a short MSL alone, without any SOREMP, should be interpreted cautiously together with the clinical picture.
Clinically, HLA-DQB1*0602 typing may be indicated when considering a CSF Hcrt measurement. Hcrt levels are generally normal if the patient is HLA-negative, unless the patient has a diencephalic lesion that explains the CNS hypersomnia (narcolepsy type 1 due to a medical condition).
5. Treatment and outcome. Treatment approaches to narcolepsy emphasize control of narcoleptic symptoms to allow optimal social and professional productivity by maintaining the patient’s alertness throughout the day. Choice of treatment must take into account that narcolepsy is a lifelong disorder and that patients will have to take medications for many years. Clinicians are not unanimous in their approach to management of narcolepsy.
a. The drugs commonly used to manage EDS and sleep attacks are the nonamphetamine stimulants (or wake-promoting agents) such as armodafinil and modafinil and CNS stimulants including methylphenidate and dextroamphetamine.
Because of the frequent side effects of sympathomimetic stimulants, such as irritability, tachycardia, elevated blood pressure, and nocturnal sleep disturbance, methylphenidate and amphetamines are probably less preferred first-line treatment. Armodafinil and modafinil have several advantages over other stimulants in that they have fewer cardiovascular side effects and longer half-lives and can therefore be taken once daily in the morning, and the prescription can be refilled.
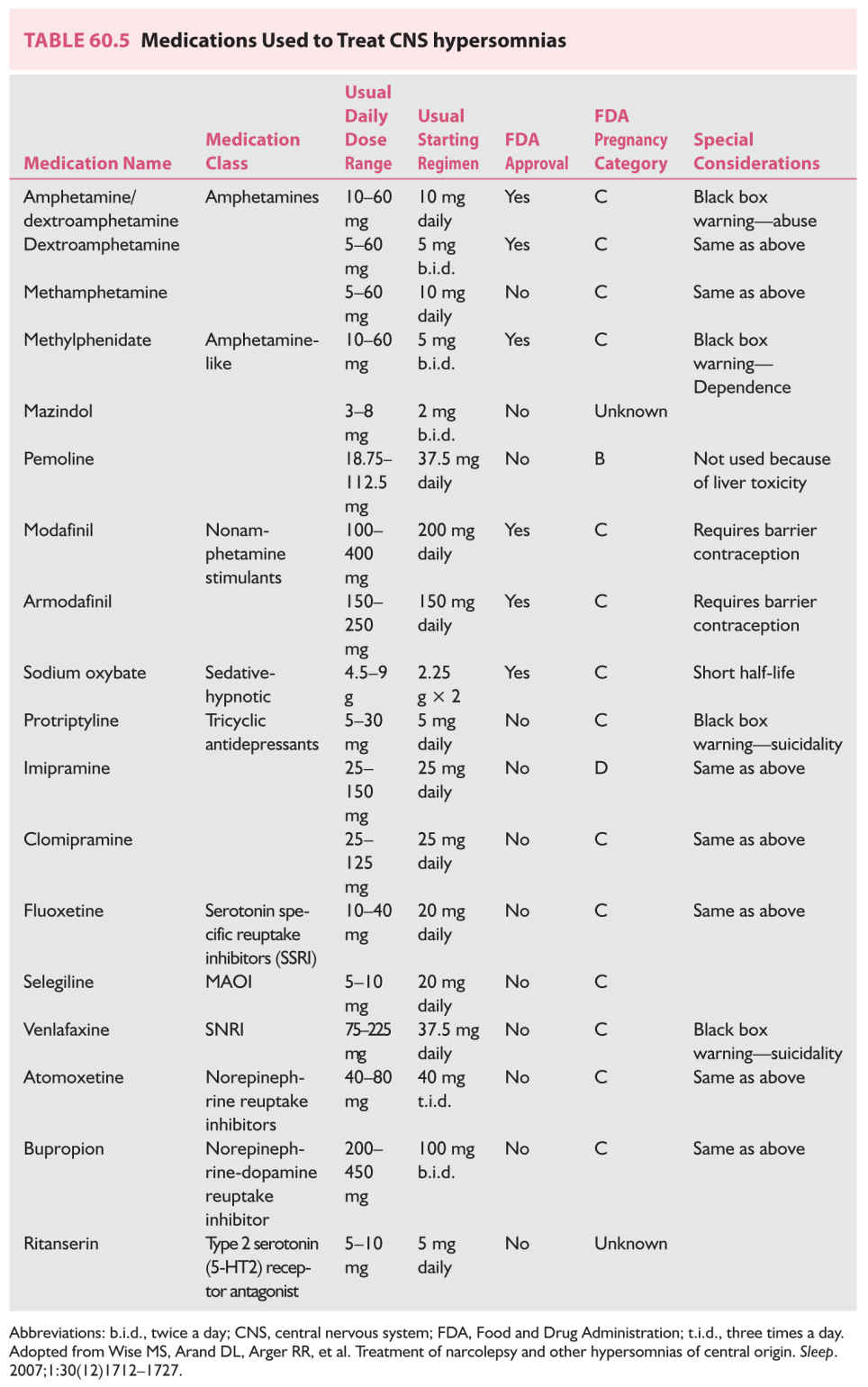
Sodium oxybate is currently the only treatment approved for the management of both symptoms of hypersomnia and cataplexy in the setting of narcolepsy. Medications used in the management of EDS and the dosages are listed in Table 60.5. Drugs with norepinephrine-releasing properties have the greatest impact on sleepiness. However, evidence shows that even at the highest recommended doses, no drug is capable of returning a person with narcolepsy to a normal baseline level of alertness.
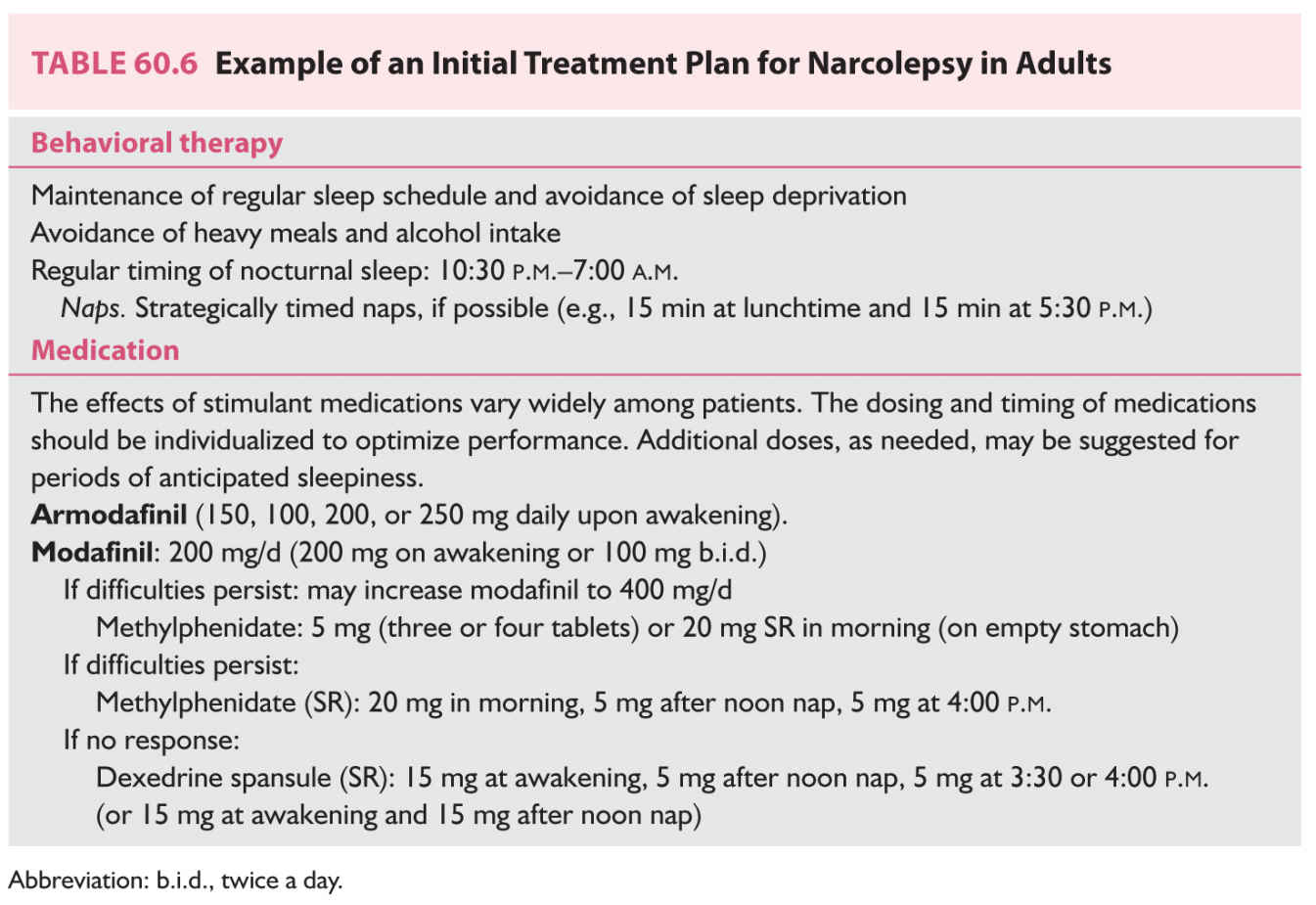
b. The management of abnormal REM intrusion phenomena such as cataplexy, SP, and hypnagogic hallucinations involves the use of sodium oxybate and tricyclic antidepressant medications. Sodium oxybate is currently approved for the management of cataplexy and daytime sleepiness in narcolepsy. Protriptyline and clomipramine have been used widely, often with good results. Other tricyclic medications, such as imipramine, desipramine, and amitriptyline, also are effective; however, anticholinergic side effects (particularly erectile dysfunction) limit the ability of many patients to tolerate these medications, particularly if high doses are needed to control cataplexy. Fluoxetine is somewhat less effective for cataplexy, but it has the advantage of being a mild stimulant (Table 60.5). An example of an initial regimen for narcolepsy among adults is provided in Table 60.6.
Status cataplecticus is an unusual state of repetitive cataplexy spells often following rapid withdrawal of anticataplectic treatment.
c. The third approach to the management of narcolepsy is to improve the nocturnal sleep of persons with narcolepsy. Improvement of nocturnal sleep not only decreases EDS but may also help address cataplexy. Nocturnal sleep disturbances may be related to periodic limb movement disorder of sleep (PLMDS), which frequently occur among patients with narcolepsy. They may, however, also be a complication of treatment with stimulants and tricyclic medications. Occasionally, management of PLMDS with dopamine agonist drug may be helpful.
d. Nonpharmacologic treatment. Scheduled short “power” naps and support therapy must be emphasized. Short naps of 15 to 20 minutes three times during the day help maintain alertness and have been shown to have a recuperative power in narcoleptic subjects.
(1) Drug holiday. In cases of tolerance, switching to a different class of medication or providing a drug holiday for 1 to 2 days can be useful.
(2) Psychosocial considerations. Patients with narcolepsy often experience social and professional difficulties owing to sleepiness and cataplexy. Narcolepsy can result in unemployment, academic difficulties, rejection by friends, and depression. For these reasons, it is important to encourage patients with narcolepsy to join support groups, like the Narcolepsy Network (http://www.narcolepsynetwork.org/), and to provide referral for psychotherapy when needed.
6. Side effects of stimulant medications. The amphetamine-like medications are typically associated with side effects such as hypertension, alterations in mood, and psychosis. Moreover, tolerance and, less frequently, addiction may be observed with drugs such as amphetamines. Interestingly, with high dosages of amphetamines (100 mg/day), a paradoxical effect of increased sleepiness may result. This paradoxical effect disappears with reduction of the daily dosage. Other common side effects include increased jitteriness, verbal aggressiveness, “racing thoughts,” increased heart rate, tremor, and involuntary movements. The most commonly reported side effects of the nonamphetamine stimulants, armodafinil and modafinil, include headache, gastrointestinal (GI) irritability, and nausea. Both modafinil and armodafinil induce the hepatic cytochrome P45 and reduce the efficacy of hormonal methods of birth control. Women of childbearing age who take these agents should switch to another form of birth control. Side effects associated with sodium oxybate include disorientation in the middle of the night and morning grogginess, enuresis, and nausea at the time of initiating the medication and at higher doses.
C. Hypersomnia other than narcolepsy.
1. Clinical course. This group of disorders characterizes patients whose diagnosis does not meet that of narcolepsy but is associated with severe disabling hypersomnia without the associated cataplexy, which is unique with narcolepsy. The age at onset varies from adolescence to middle age. The symptoms are lifelong, with some potential for improvement if an associated condition is identified.
2. Clinical features. Patients report sleepiness throughout the day associated with prolonged naps, which, unlike narcolepsy, are not refreshing. Automatic behaviors and some features of REM sleep intrusion events (such as hypnogogic hallucinations) may occur during periods of drowsiness. These behaviors are often inappropriate, and patients usually do not have any recollection of these events. Patients have severe difficulty awakening in the morning.
3. Examples of hypersomnia other than narcolepsy.
a. Idiopathic hypersomnia (IH) is characterized by greater than 3-month duration of EDS and an irrepressible need to sleep or daytime lapses into sleep in the absence, and after correction, of sleep deprivation.
The onset of the disease is generally around the same age as narcolepsy (15 to 30 years). The sleep pattern, however, is different from that of narcolepsy. The patient generally sleeps for hours, but the sleep is not refreshing. Total 24-hour sleep time is typically 12 to 14 hours. The MSLT shows a mean sleep-onset latency of 8 minutes or less, and unlike narcolepsy, with less than two SOREMPs. Supportive features also include profound sleep inertia (aka sleep drunkenness, elucidated as prolonged difficulty waking up accompanied by irritability and repeated returns to sleep), dependence on others for awakening them, mental fatigability, and often prolonged (>60 minutes), unrefreshing naps. As part of the sleep drunkenness spectrum, some patients may have automatic behavior with amnesia for the events.
The patient suffering from IH does not endorse a history of cataplexy, snoring, or repeating awakenings throughout the night. Physical examination uncovers no abnormal neurologic findings. This disabling and lifelong condition should be differentiated from other causes of EDS. REM intrusion phenomena such as SP and hypnagogic hallucinations may also be reported, but these are far less frequent than in narcolepsy and without clear association between IH and HLA. It is indeed very interesting that NT-1 may represent a completely separate and unique phenotype of hypersomnolence, exemplified by HRCT deficiency and cataplexy in HLA-susceptible individuals, which is distinct from other central forms of hypersomnia such as NT-2 and IH.
b. Kleine–Levin syndrome (KLS aka recurrent hypersomnia, periodic hypersomnolence): This is a very rare condition in which patients experience prolonged episodes of severe sleepiness separated by periods of normal alertness and function. The ICSD-3 requires that there be at least two episodes of EDS lasting between 2 days and 5 weeks with periods of normalcy between episodes (normal alertness, cognition, and mood). KLS usually affects adolescent males, who also experience cognitive impairment, hyperphagia, megaphagia, and disinhibited behavior manifesting as hypersexuality. During the episodic sleep attacks, it is not uncommon for patients to sleep for 16 to 18 hours a day, eat voraciously while awake, and experience other behavioral disturbances during the episodes, including confusion, hallucinations, hyperorality, memory impairment, and polydipsia. The hypersomnia should not be better explained by another disorder, especially bipolar disorder.
D. Hypersomnia caused by a medical condition. Hypersomnia may be diagnosed when sleepiness is thought to be the direct result of a medical or neurologic condition, but the patient does not meet clinical or laboratory criteria for a diagnosis of narcolepsy. A variety of conditions may underlie this disorder, including associated neurologic disorders such as encephalitis, cerebrovascular accidents, brain tumor, head trauma, and Parkinson’s disease. Common genetic conditions associated with sleepiness include Prader–Willi syndrome and myotonic dystrophy.
E. Diagnosis. The differential diagnosis includes narcolepsy and primary sleep disorders such as sleep-disordered breathing or periodic limb movement in sleep (PLMS), which may also be associated with significant daytime sleepiness. Therefore, the diagnosis is made by means of elimination of other causes of daytime sleepiness. Polysomnography should be performed to further assess these possibilities, and MSLT should be performed to document the level of objective daytime sleepiness.
Mean sleep latencies are often less than 8 minutes but unlike narcolepsy, which is diagnosed electrographically when the MSL is <8 minutes and when two or more SOREMPs are present, the criteria for the latter must include less than two SOREMS and an equally short sleep latency.
F. Treatment and outcome. Because of multiple etiologic factors and the relative lack of understanding of the underlying pathophysiologic mechanism, treatment is symptomatic and the response is variable.
Behavioral therapies and sleep hygiene instructions should be recommended but have only modest positive effect. The only medications that provide partial relief of excessive sleepiness are stimulant-like drugs. The most commonly suggested medications are armodafinil, modafinil, sodium oxybate, methylphenidate, and dextroamphetamine. Tricyclic antidepressants, selective SSRIs, clonidine, bromocriptine, amantadine, and methysergide have been used with varying success.
Sometimes combinations of these drugs yield better control of sleepiness. Even with the highest recommended dose, complete control of daytime sleepiness is seldom achieved in this group of patients. Therefore, prescribing more than 400 mg of modafinil, more than 60 mg of methylphenidate, or 40 mg of dextroamphetamine does not provide significant additional symptomatic relief. The patient should be advised not to drive or engage in potentially dangerous activities that require high levels of alertness. Pemoline is not recommended because of its potential hepatotoxicity (see Table 60.5).
Treatment for patients with KLS includes the amphetamine and nonamphetamine stimulants, and mood stabilizers such as lithium (probably most effective), valproic acid, and carbamazepine.
PARASOMNIAS
Parasomnias are a group of disorders that occur during sleep, are associated with abnormal wake-to-sleep transition, or abnormal arousal from sleep. These conditions with important consideration in neurology include REM sleep behavior disorder (RBD), sleepwalking (somnambulism), night terrors, nightmares, and confusional arousals. The ones that are most often encountered in adult clinical practice are discussed in this chapter.
A. Non-REM parasomnias (aka disorders of arousal, DOA). Examples include confusional arousals, sleepwalking (somnambulism), and sleep terrors (pavor nocturnus). They represent episodic arousals from non-REM sleep, typically slow-wave sleep, associated with amnestic behaviors.
1. Clinical course. The prevalence of sleepwalking and sleep terrors is estimated at approximately 6% of the population. These types of parasomnias are most frequent among children and often disappear by adolescence. These behaviors may be considered normal among children, but abnormal when they persist into adulthood, which may be related to sleep deprivation, underlying comorbid medical, sleep, or psychiatric conditions, and CNS-acting medications.
2. Clinical features. During these episodes, a variety of behaviors may occur ranging mild to severe: patients will exhibit a state of prolonged confusion with uttering irrelevant words (confusional arousals), sitting up, and getting up to walk (somnambulism/sleep walking), and may experience extreme autonomic sympathetic discharge culminating with screaming and sometimes aggression (sleep terrors). These episodes have the potential to become dangerous because patients may bump into walls and windows or fall down stairs. With all of these episodes, patients usually have only vague recollections of these events and are confused or agitated if awakened.
a. Unique subtypes.
(1) Sleep sex (aka sexsomnias). This is a variant of confusional arousals where patients experience automatic and inappropriate amnestic sexual behavior, out of character for the patient. It may be related to underlying untreated primary sleep disorders (i.e., sleep-disordered breathing, sleep deprivation).
(2) Sleep-related eating disorders. The condition is a variant of sleepwalking and is common in younger women who are given a hypnotic agent. Episodes consist of recurrent episodes of involuntary eating and drinking during partial arousals from sleep without recollection/very partial recall. The eating behavior is sometimes unusual for the types of food items consumed (i.e., jam and cat food sandwich). The episodes may lead to weight gain and sometimes injury during food preparation. The condition can be either idiopathic, but occasionally in association with Willis–Ekbom Disease (WED) and use of CNS medications such as zolpidem.
3. Diagnosis. For adults, thorough evaluation of abnormal nocturnal behavior should be performed to differentiate non-REM parasomnias from other pathologic entities, particularly nocturnal seizures and REM parasomnias. The most common PSG findings are multiple abrupt arousals, arising predominantly from slow-wave sleep, but also from other stages of NREM sleep associated with the behavior, such as confusion, eating, walking, sympathetic hyperarousal. When ordering PSG in patients who may have parasomnia, it is important to arrange for the study to include expanded four-limb electromyographic (EMG) montage with high-definition video monometry, and ensure that the sleep laboratory is aware to ask patients about recollection of their spells, while ensuring that the monitoring environment is safe (monitoring bed is padded). Expanded EEG montage may also be utilized with nocturnal seizures on the differential diagnosis (especially for repetitive and stereotyped nocturnal spells) ![]() (Video 60.1).
(Video 60.1).
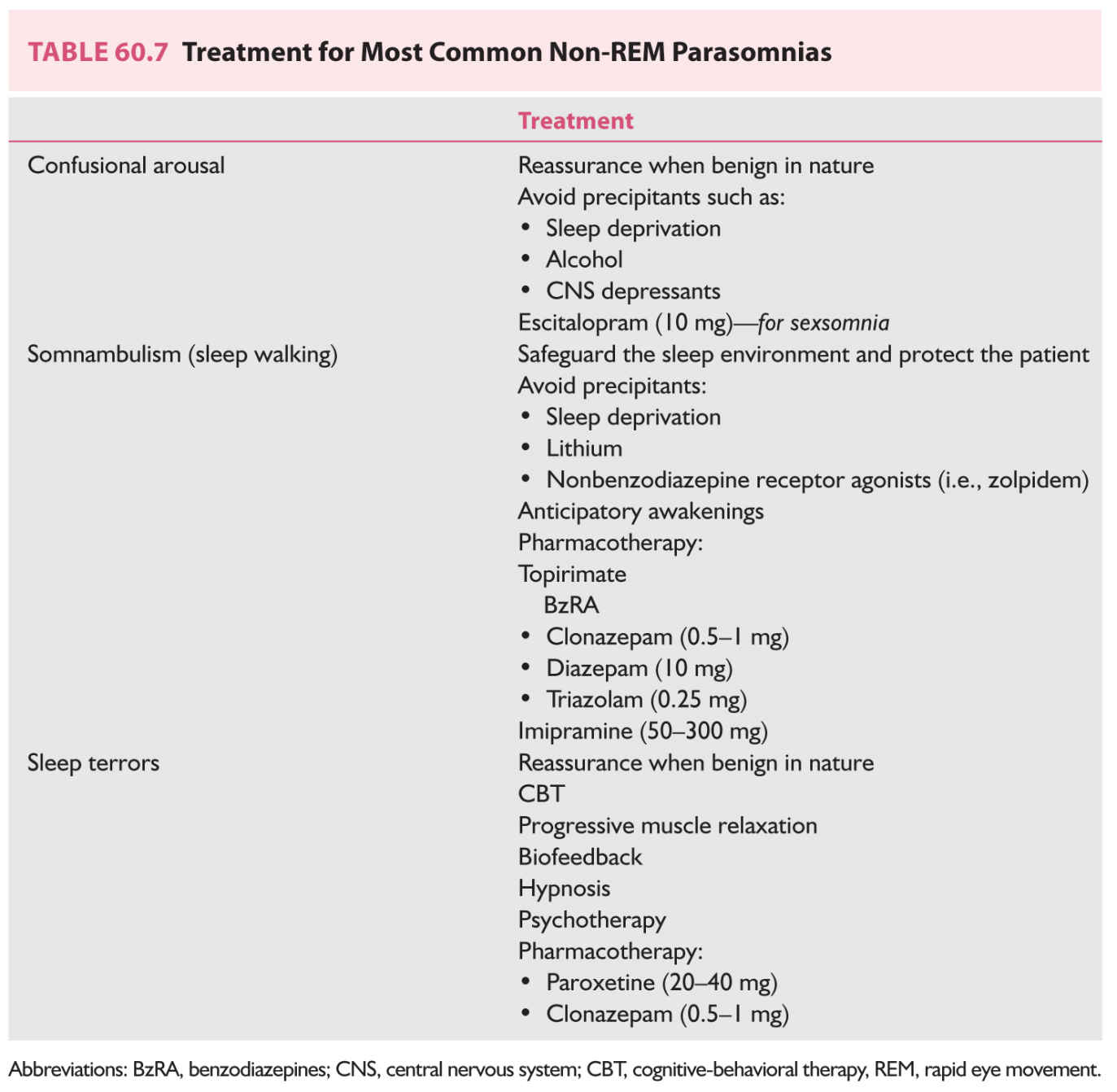
4. Treatment and outcome. Therapy for non-REM parasomnias includes several approaches consisting of maximizing safety measures around the bedroom area (i.e., removal of sharp furniture, placing the mattress on the floor), preventive measures (avoidance of sleep deprivation, caffeine), psychological interventions, and when frequent/disruptive/severe, using medications (Table 60.7).
a. Preventive measures and psychological intervention. Preventive measures are taken to avoid serious injury during episodes of sleepwalking. The patient should be advised to locate the bedroom on the ground floor, lock windows and doors, place door alarms, cover windows and glass doors with heavy draperies, and remove hazardous objects (sharps, knives, firearms) from the house.
Comorbid sleep disorders such as sleep apnea and sleep deprivation should be addressed. Caffeine should be minimized. Although alcohol remains a known precipitant for some DOA, its specific role in provoking somnambulism is somewhat unclear/controversial.
Hypnosis and psychotherapy have also been used in the management of parasomnias. Hypnosis has been shown helpful, at least for a short time, to young adults. The need for psychotherapy depends on the association of psychological factors with the parasomnia. Psychotherapy has been used most widely to treat young adults for sleep terrors. Most cases of parasomnia increase in severity and frequency with psychological stress. Therefore, in addition to psychotherapy, relaxation programs, such as progressive muscle relaxation and biofeedback, may be beneficial. Anticipatory awakening has been reported as a treatment modality for sleepwalking and perhaps other DOA. The technique involves waking the patients between 15 and 30 minutes prior to the time of the typical episodes.
b. Medications. The BzRA—most commonly clonazepam, alprazolam, and diazepam—have been used. In the management of sleep terrors, tricyclic antidepressants (particularly imipramine) have been used either alone or in combination with BzRA to provide control of symptoms. In addition, several studies have shown that treatment with carbamazepine may be beneficial. An example of an initial therapeutic approach is to start with clonazepam (0.25 to 1 mg) approximately 30 minutes before bedtime. If the response is inadequate, the dose may be increased while balancing the side effects, which include confusion and daytime drowsiness. A secondary line of treatment includes initiation of low doses of tricyclic antidepressant drugs.
Results of management of non-REM parasomnias are poorly documented. However, the literature indicates that response to combinations of pharmacologic and nonpharmacologic therapies is generally good. After various lengths of time, as many as 70% of adult patients report improvement of the symptoms.
This group consists of three important parasomnias, but one in particular, RBD, is of particular importance for neurologists.
1. RBDs.
a. Clinical course. REM sleep and dreaming is normally accompanied by skeletal muscle atonia. RBD is characterized by abnormal loss of REM-sleep atonia leading to excessive motor activation during sleep. Patients with this disorder most commonly experience vigorous and often aggressive dream behaviors that are accompanied by vivid dreams in which the patient is usually attacked by an intruder or animals. These behaviors may be quite violent and can result in serious injury to both patient and bed partner. Two specific RBD phenotypes may exist—a chronic form and an acute form. The chronic form usually occurs among older adults (>65 years of age) and is most common in male patients (having a 9:1 male-to-female predilection). It is encountered in association with alpha-synucleopathies such as Parkinson’s disease and Parkinson’s plus syndrome (multiple systems atrophy, Lewy body dementia). The acute form is usually associated with toxic–metabolic etiologic factors, most commonly, drug withdrawal states and antidepressant medications [i.e., antidepressants such as SSRI, SNRI, TCA, monoamine oxidase inhibitors (MAOIs)], various brainstem abnormalities, brainstem stroke, brainstem tumor, and demyelinating disease. The acute form is usually more common in younger (<50 years old) patients and does not have the gender predilection favoring males in the chronic form. Loss of REM EMG tone may also occur among patients taking medications that suppress REM sleep, such as tricyclic antidepressants and fluoxetine, and substances such as caffeine.
RBD may be idiopathic or secondary; however, it is currently believed that most patients most likely represent the secondary form and some have advocated replacing the term “idiopathic” with “cryptogenic” because of the high-degree phenoconversion to neurodegenerative diseases.
Besides the need to provide efficacious treatment for this potentially injurious REM parasomnia, the most important reason to properly diagnose this condition relates its strong prognostic utility as a predictor of neurodegeneration. RBD generally precedes the onset of the alpha-synucleinopathy by a few years to decades. Reviewing the underlying neurodegenerative disorders associated with RBD, 94% of recently reported cases were in the setting of alpha-synucleinopathies. The recent data on the rates of RBD phenoconversion to the emergence of a neurodegenerative disorder may exceed 90% over a 14-year observation period.
The differential diagnosis of RBD includes non-REM parasomnias, severe obstructive sleep apnea (“pseudo RBD”), and periodic movements of sleep, nocturnal seizures, and nocturnal rhythmic movements. It is important to recognize this condition and differentiate it from other nocturnal behaviors because RBD can be managed effectively.
Recent data demonstrate several potential markers of neurodegenerative diseases in idiopathic RBD (before the onset of dementia diagnosis) including impaired cognition, visuospatial dysfunction, impaired color vision, anosmia, and autonomic dysfunction, particularly orthostatic hypotension. Interestingly, for patients who have RBD but normal olfaction, the risk of developing a neurodegenerative disease over the next 5 years is approximately 15%. If anosmia is present, however, that risk increases dramatically to 65%.
b. Diagnosis. A diagnosis of RBD should be suspected in patients with a clinical history of recurrent dream-enactment behavior (DEB), but must be confirmed with polysomnography. The clinical evaluation should include a detailed interview of the patients, but equally, if not more important, the bed partner, as some patients are unable to adequately describe/recall the sleep-related events. One key question to ask the bed partner is “Have you ever seen the patient appear to “act out his or her dreams” (punched or flailed arms in the air, shouted, or screamed) while sleeping?”
As opposed to the DOA, a unique feature that may further differentiate RBD from other nocturnal spells is the timing of the episodes in the latter part of the night, where a greater REM occupies a larger percentage of the sleep cycle.
In patients who experience DEB, the diagnostic polysomnography is mandated for a definitive diagnosis to exclude DOA, nocturnal seizures, periodic limb movements, and sleep apnea (“pseudo RBD”). The presence of DEB by history/during the PSG along with evidence of RSWA is essential for diagnosis.
The ICSD diagnostic criteria for RBD require all of the following to be present:
i. Repeated episodes of sleep-related vocalization and/or complex motor behaviors
ii. The behaviors are documented by PSG during REM sleep or, based on clinical history of dream enactment, are presumed to occur during REM sleep.
iii. PSG recording confirms the presence of RSWA. The muscle augmentation may be based on recording from the limbs or chin EMG.
iv. The disturbance is not better explained by another sleep disorder, mental disorder, medication, or substance use.
c. Treatment and outcome. Management of RBD should begin with interventions that target environmental safety, and when appropriate proceed with pharmacologic therapy. When appropriate, disclosure of the risk for neurodegeneration should take place. The decision to treat and the specific treatment to select are based on a number of factors—frequency and severity of episodes and comorbidities such as sleep apnea.
(1) Environmental safety provides for the strongest level of evidence for managing RBD (level A evidence). Patients with frequent episodes of motor behavior should be advised to remove potentially dangerous objects from the bedroom, to pad hard and sharp surfaces/furniture around the bed, to cover windows with heavy draperies, and even to place the mattress on the floor, or to sleep in a sleeping bad, to avoid injury until pharmacotherapy is successful.
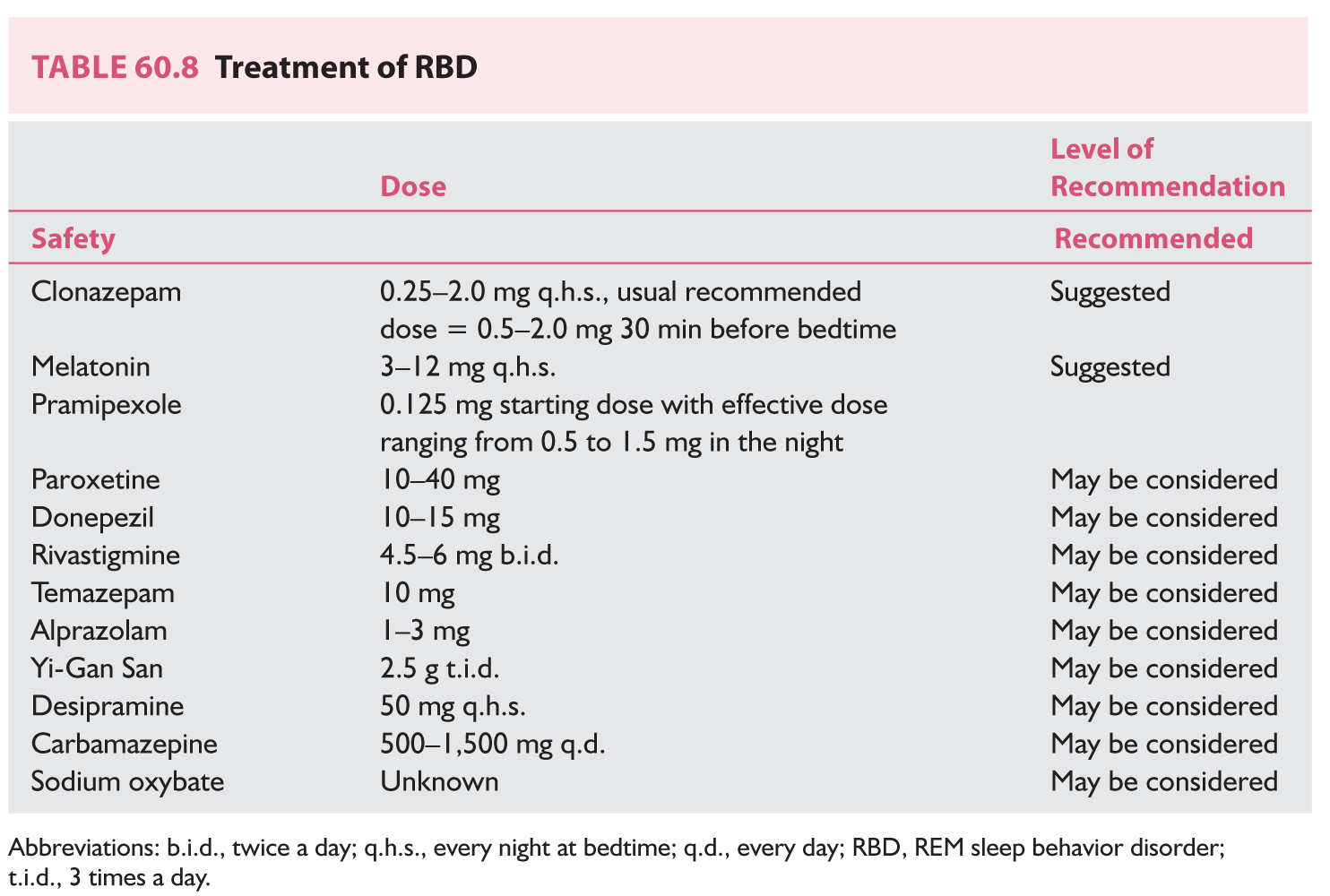
(2) The most commonly prescribed drug therapies include either clonazepam at a dosage of 0.25 to 2.0 mg, or melatonin at 3 to 12 mg at bedtime. If patients have significant OSA associated with RBD, they should be treated with positive pressure therapy because clonazepam may potentially exacerbate OSA, or be given melatonin instead. Clonazepam is effective in 90% of cases, and there is little evidence of abuse and infrequent in tolerance in this group of patients. Beneficial effects are observed within the first week of treatment. Typically, treatment with clonazepam results in control of vigorous, violent sleep behaviors, but mild to moderate limb movement. Discontinuation of treatment usually results in recurrence of symptoms.
Melatonin (3 mg titrated to clinical efficacy up to 12 mg q.h.s.) has been shown to be useful in improving DEB and has the added advantage of restoring muscle atonia. Melatonin may be advantageous over clonazepam because of its relative lack of sedation, cognitive impairment, and lack of respiratory suppression. When either melatonin or clonazepam fail to control the behavioral spells or disruptive hallucinations, both may be sued simultaneously, or the patient may be placed on a different therapy. Table 60.8 outlines the treatment options for RBD with the respective level of evidence based on a recent literature review. Both melatonin and clonazepam appear to have the strongest level of evidence in the management of this condition.
Although no specific treatment is available to prevent phenoconversion to neurodegenerative disease at present, RBD represents a unique opportunity to someday in the future provide a potential biomarker for selecting appropriate patients to be treated with neuroprotective agents to slow down or even prevent the conversion to neurodegeneration.
2. Recurrent isolated SP. Intrusion and persistence of muscle atonia of REM sleep into wakefulness is a central feature. Clinically, patients are unable to move or speak and experience a profound sense of impending doom and anxiety. As noted in the narcolepsy section, SP can occur as part of the classic narcolepsy pentrad, but in most individuals, SP is typically an isolated symptom and is specifically related to sleep deprivation. Specific treatment for SP is not needed in most patients as avoidance of sleep deprivation is typically effective, and some patients with recurrent episodes unrelated to sleep loss may be managed with REM-suppressing therapy (such as SSRIs).
3. Nightmare disorder. The most prominent feature here consists of distressing visual imagery during REM sleep, with persistence into wakefulness. Unlike patients with sleep terrors, who are typically amnestic about event, those experiencing nightmares typically have good recollection of the episode, are able to detail their dream, but typically lack the abrupt and heightened autonomic hyperarousal and the episodes are not aggressive and lack motor activity.
Although specific pharmacotherapy of nightmares is usually not needed, patients with recurring and fearful nightmares may be managed with combined behavioral or psychotherapy and REM sleep-suppressant medications. Prazosin is helpful in reducing the severity and frequency of nightmares associated with posttraumatic stress disorder.
MOVEMENT DISORDERS OF SLEEP
A. RLS and PLMDS.
1. Clinical course. RLS, more appropriately referred to as WED (Willis-Ekbom disease), is characterized by a compulsive urge to move the lower, and occasionally in the upper, extremities, associated with irresistible movements of the extremities. The symptoms are often described by patients as creeping, crawling, and disagreeable sensations in the limbs, worse at rest (quiesogenic), and are relieved by movements such as stretching, rubbing, and walking. Lying down in bed and falling asleep is a major problem for patients with RLS. The need to move the lower extremities has a circadian predilection to be most severe at bedtime, and is often associated with sleep-initiation insomnia, which is often the most common reason for presentation. RLS must be differentiated from other conditions that could mimic RLS and clinically significant RLS is defined by symptoms leading to significant distress, sleep disturbance (insomnia/hypersomnia), or impairment of daily function.
Up to 80% of patients with RLS also have PLMS. However, PLMS can occur without RLS and has its own diagnostic category. Unlike RLS, which is a clinical diagnosis, PLMS is suspected when a patient or bed partner reports repeated leg kicks and is confirmed by periodic limb movements in the PSG. The typical PSG findings consist of stereotyped repetitive rhythmic movements. The leg movement must last for 0.5 to 10 seconds, and candidate leg movements are considered “periodic” if three or more occur with their onsets separated by 5 to 90 seconds. The leg movements consist of dorsiflexion of the foot, and occasionally may also involve the upper extremities. PLMS is usually more frequent during the first half of the night but can be present throughout sleep. Movements may be associated with sleep disruption. If numerous, the movements can result in nocturnal awakenings and EDS. The prevalence of PLMS increases with age, from 5% among those younger than 50 years to 44% among those 65 years and older. A PLM index is calculated by dividing the total number of periodic limb movements by the total sleep time (in hours), providing an average number of periodic limb movements per hour of sleep time. PLMS may be diagnosed if the patient has a PLM index greater than 15 per hour, in adults, or 5 per hour in children. PLMS is found out by PSG EMG, whereas an individual must report a sleep disturbance or a specific functional impairment that is causally related to the periodic limb movements, in which case the condition of termed PLMD. It is important to establish a cause-and-effect relationship between the insomnia or hypersomnia and the PLMS. The clinician should verify that other causes of insomnia (i.e., anxiety) or hypersomnia (i.e., narcolepsy, sleep apnea) are ruled out. PLMS is common but PLMD is believed to be less common in adults.
For most patients with RLS or PLMD, the cause is unknown and therefore termed idiopathic. In many cases, RLS is familial and has an autosomal-dominant inheritance with a significant genomewide association with a common variant in an intron of BTBD9 on chromosome 6p21.2, in addition to other gene variants (MEIS1, MAP2K5/LBXCOR, and PTPRD). Both RLS and PLMD are associated with anemia resulting from iron deficiency, particularly when the ferritin level is <45 mcg/L. Folic acid, vitamin B12 deficiency, neuropathy, myelopathy, rheumatoid arthritis, thyroid dysfunction, and uremia have also been shown to have an association. Results of studies suggest that RLS is associated with alteration in cerebrospinal fluid ferritin levels. Furthermore, PLMD may be induced or exacerbated by SSRIs and tricyclic antidepressants as well as by withdrawal from a variety of hypnotic agents. The existence of these conditions should be entertained in the differential diagnosis of PLMD so that patients receive the appropriate therapy. If these conditions are suspected, referral to the appropriate specialist is recommended.
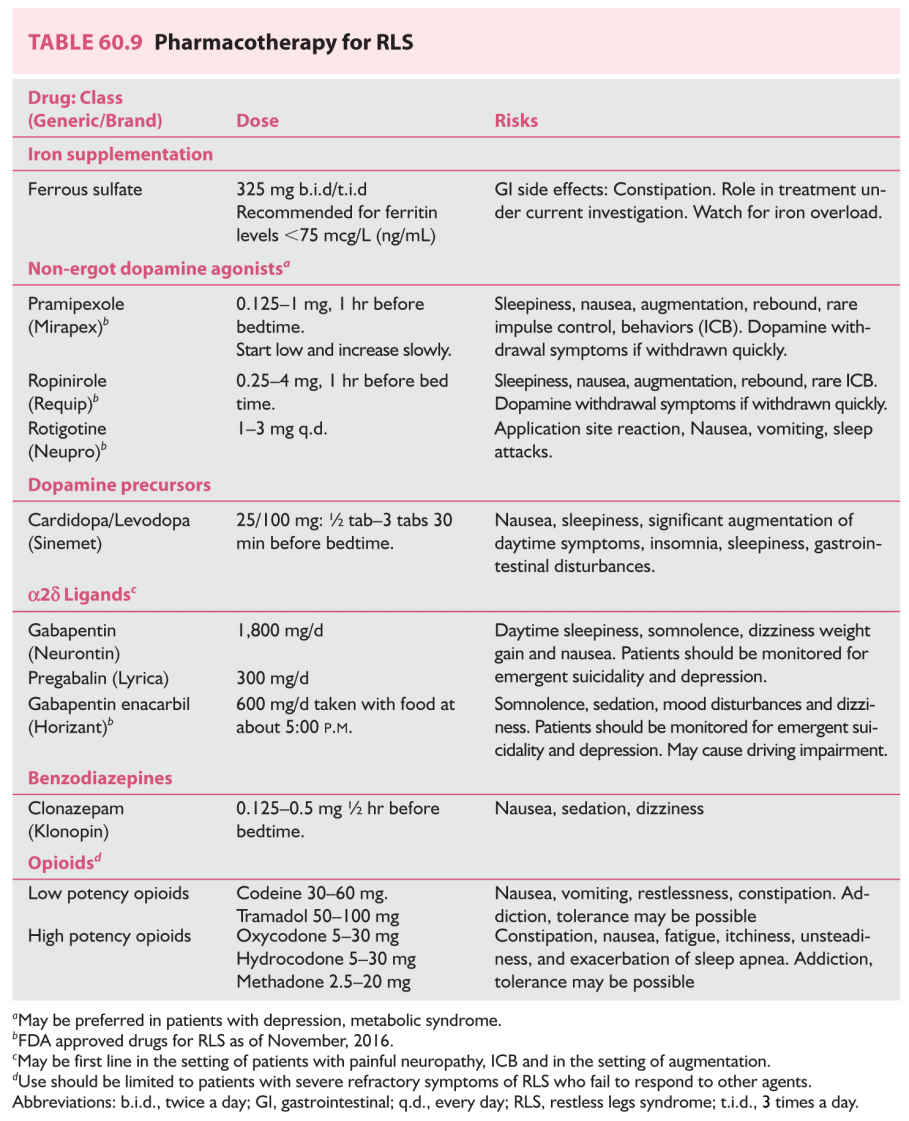
2. Treatment. The four major classes of drugs that have been shown to be effective in the management of RLS are dopaminergic drugs, alpha-2-delta (a2d) ligands, BzRA, and opioids. Common medications used in the treatment of RLS are shown in Table 60.9.
a. The first approach for patients with symptoms consistent with that of RLS is to draw a serum ferritin level. Patients with levels less than 45 mg/L should begin iron replacement therapy with iron sulfate along with vitamin C to improve absorption.
b. When iron stores are normal, nonergotamine dopamine (D2 D3) agonists such as rotigotine, ropinirole, or pramipexole may be started. Two a2d ligands, pregabalin and gabapentin enacarbi, have been shown to be effective in patients with primary moderate-to-severe RLS with painful symptoms and sleep disruption. Three dopamine agonists (rotigotine, ropinirole, and pramipexole) and one a2d ligand, gabapentin enacarbi, are specifically FDA approved for the treatment of RLS. Major side effects include unpredictable sleep attacks, GI side effects, postural orthostatic hypotension, and, at higher doses, rare reports of impulse control behaviors (compulsive gambling). Patients on chronic dopamine therapy may experience augmentation, defined as a worsening of RLS symptoms earlier in the day with geographic spread to body parts other than the legs. Rebound may also occur and is characterized by a reappearance and worsening of symptoms as the effects of a medication wear off between doses.
c. Historically carbidopa/levodopa (at a dose of 25 mg/100 mg q.h.s.) was initially widely used. However, its wide use is significantly limited because of the risk of rebound symptoms as well as frequent augmentation rates (up to 30%). It is better used in patients who require “on-demand” therapy of their RLS, such as when driving/flying long distances.
d. The a2d ligands, pregabalin and gabapentin enacarbi, of moderate-to-severe primary RLS in adults are not associated with impulse control behaviors or augmentation and are effective when patients experience pain, anxiety, or insomnia as part of their RLS. Therapy, however, is sometimes limited by sedation, weight gain, and depression.
e. Several BzRA, including clonazepam, nitrazepam, lorazepam, and temazepam, have been found to improve the nocturnal sleep of patients with RLS and PLMS. Of these, clonazepam is the most widely used. The therapeutic action of clonazepam most likely results from its ability to decrease the number of arousals caused by leg movements. The usual starting dosage of clonazepam is 0.5 to 1.0 mg at bedtime for management of PLMS. Management of RLS may require additional doses to control symptoms during the day. Because BzRA are CNS depressants, they may aggravate sleep apnea, particularly among older persons.
f. Finally, opioids are highly effective in the management of RLS and PLMS. In severe cases refractory to other treatments, intermittent therapy with opioids provides good relief without evidence of augmentation.
The most commonly encountered types of abnormal nocturnal breathing are the sleep apnea and hypopnea. Sleep apnea is cessation of breathing for at least 10 seconds caused by obstruction of the upper airway (obstructive sleep apnea), loss of respiratory effort or rhythmicity (central apnea), or a combination of the two (mixed apnea). Hypopnea is a decrease in airflow, which can be obstructive or central in origin. Many patients with sleep apnea have combinations of the central and obstructive types, which suggest that the mechanisms of the different types of sleep apnea may overlap.
A. Central sleep apnea (CSA).
1. Clinical course. Patients with CSA constitute less than 10% of all patients with sleep apnea who undergo studies in sleep laboratories. Therefore, only a few studies have been reported, which limits knowledge of this disorder. Little information is available regarding the cardiovascular sequelae of CSA. The most common finding is sinus arrhythmia with bradycardia. Oxygen desaturation in patients with CSA tends to be generally mild to moderate compared with those with obstructive sleep apnea. Although the cause of CSA in most cases is unknown, it has been associated with certain diseases that should be considered in the differential diagnosis and management of this disorder. These diseases include central alveolar hypoventilation (Ondine’s curse), obesity hypoventilation (Pickwickian) syndrome, congestive heart failure (Cheyne–Stokes breathing pattern), autonomic dysfunction (Shy–Drager syndrome, familial dysautonomia, and diabetes mellitus), neuromuscular disorders (muscular dystrophy, myasthenia gravis, and motor neuron disease), and brainstem lesions.
2. Treatment of patients with CSA is limited and not satisfactory. Studies regarding treatment usually have involved small numbers of patients, and very few have addressed the long-term efficacy of the proposed treatments.
a. One approach is noninvasive nocturnal ventilation delivered by means of a nasal mask with a volume- or pressure-cycled ventilator. This approach is used to manage only the most severe cases of central alveolar hypoventilation or in the care of patients with neuromuscular disorders.
b. Some patients with CSA have been shown to benefit from therapy with nasal continuous positive airway pressure (CPAP). This type of therapy is most beneficial to obese patients who also have signs of upper airway obstruction with predominantly central apnea. Nasal CPAP also has been shown effective in the treatment of patients with congestive heart failure in whom central apnea and periodic breathing are observed during sleep. Finally, oxygen therapy has been useful in managing central apnea.
c. Adaptive seroventilation (ASV): ASV provides a relatively low baseline pressure and variable ventilatory support to establish a preset level of ventilation for each breath. If the patient’s effort decreases, the ASV’s inspiratory support increases to maintain a steady level of ventilation. This treatment is indicated in patients with CSA, but also OSA patients are refractory to standard CPAP. The term treatment-emergent sleep apnea (formerly known as “complex sleep apnea”) refers to patients with OSA who develop CSA on initiation of CPAP. These patients with so-called treatment-emergent central apneas often experience spontaneous resolution of their disease with ongoing therapy.
The reader needs to be aware that ASV is associated with an increased risk of cardiovascular mortality in the setting of symptomatic chronic heart failure with reduced ejection fraction (EF) [low EF (≤45%)].
d. For patients with medical or neurologic conditions known to be associated with CSA, the condition should be managed specifically and the central apnea reassessed. However, if the problem persists or if a cause is not found, several pharmacologic agents can be used. Acetazolamide, a carbonic anhydrase inhibitor, given at a dose of 250 mg four times a day, has been shown to improve CSA. The side effects associated with mild metabolic acidosis are usually well tolerated by this group of patients.
e. Other medications, such as theophylline, naloxone, and medroxyprogesterone acetate, have been used with varying degrees of success. Because none of these medications has been studied systematically, more precise recommendations regarding their use are currently not available.
B. Obstructive sleep apnea (OSA).
1. Clinical course. The initial symptoms of OSA syndrome are loud snoring, excessive sleepiness, fatigue, morning headaches, memory problems, alterations in mood, and episodes of apnea witnessed by the bed partner. OSA is associated with considerable morbidity, including sleep fragmentation, daytime sleepiness that may lead to vehicular and industrial accidents, nocturnal hypoxemia, and cardiovascular as well as cerebrovascular sequelae (e.g., stroke, right heart failure, and hypertension). OSA is generally caused by upper airway obstruction resulting from obesity and skeletal and soft-tissue abnormalities. Examination of the nose and throat may indicate a possible cause. However, some patients with OSA may have normal findings at physical examination.
If OSA is suspected, PSG should be performed to ascertain the severity of the breathing disorder, which will determine the appropriate therapy. Some patients who have symptoms indistinguishable from those of OSA may have predominantly sleep hypopnea. Sleep hypopnea syndrome should be managed in the same manner as sleep apnea syndromes.
The apnea–hypopnea index (AHI) (number of respiratory events per hour of sleep) is used to measure sleep-disordered breathing. An index of 5 is generally accepted as the upper limit of the normal range. Patients who have milder indexes but whose respiratory events are accompanied by more significant oxygen desaturations and who have additional cardiovascular risk factors such as hypertension, history of heart disease, high cholesterol level, and cigarette smoking also should be treated.
In adults, a diagnosis of OSA is defined by either of the following:
a. AHI >5, in a patient with one or more of the following:
(1) Sleepiness, nonrestorative sleep, fatigue, or insomnia symptoms
(2) Waking up with breath holding, gasping, or choking
(3) Habitual snoring, breathing interruptions, or both noted by a bed partner or other observer
(4) Hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus
b. AHI >15, regardless of the presence of associated symptoms or comorbidities.
2. Therapy. The approach to management of obstructive sleep apnea and hypopnea syndromes involves both general measures and interventions that address specific abnormalities. For most patients, nasal CPAP is the most effective medical therapy for control of sleep apnea.
a. General measures for identifying and addressing coexistent lifestyle issues that exacerbate OSA should be part of treatment of all patients. Although difficult to achieve, weight loss is an important factor in the treatment of obese persons with apnea. Sleep apnea generally improves with weight loss and may even be improved with weight loss of 40 to 50 pounds (18 to 23 kg). In addition to dietary control, this approach requires an exercise program and psychological counseling for long-lasting results. Unfortunately, results indicate that most patients regain the weight within 2 years. If sleep-disordered breathing is more prominent in the supine position, positional therapy to avoid sleep in the supine position is very useful. Alcohol, hypnotic drugs, and other CNS depressant drugs interfere with the arousal response that terminates apneic episodes. Therefore, patients should avoid alcohol use and should not take hypnotics or sedatives. If a specific cause for upper airway obstruction is found, an otorhinolaryngologic or maxillofacial evaluation is recommended for possible surgical intervention and trials of orthodontic devices, including tonsillectomy or adenoidectomy for enlarged tonsils or adenoids and correction of retrognathia or micrognathia. Results indicate that dental devices may be useful to those patients with mild-to-moderate sleep apnea with some degree of retrognathia or micrognathia. If chronic rhinitis is found, nasal steroid sprays may be beneficial.
b. Nasal CPAP. If no specific cause of upper airway obstruction is found, nasal continuous positive airway pressure (CPAP) is the treatment of choice. This treatment is effective for most patients with obstructive apnea and hypopnea. The level of CPAP should be determined by means of titration of the therapeutic pressure in a sleep laboratory, respiratory data being obtained in all sleep stages. Nasal CPAP requires patency of the nasal airway. Therefore, this procedure may not be effective for patients with severe nasal obstruction. The most common causes of intolerance of nasal CPAP are nasal symptoms, dryness, discomfort from the mask, and social and psychological factors of having to use the mask during sleep (because of claustrophobia). Added humidification often alleviates dryness and associated nasal congestion. With higher pressures, bilevel positive airway pressure (BiPAP) may be a more comfortable alternative to CPAP. Most home-care companies provide both nasal CPAP and BiPAP services. If a patient with sleep apnea also has low baseline oxygen saturation during the day or during sleep, referral to an internist or pulmonologist is recommended. Although improvement of symptoms, including daytime sleepiness, may be observed within 1 or 2 days of treatment with nasal CPAP, maximal improvement may not occur for several weeks. Follow-up studies indicate that long-term compliance with nasal CPAP is a substantial problem for many patients not using CPAP throughout the night and on a daily basis. Compliance increases with close follow-up care. Follow-up visits should be scheduled 1 month after the start of CPAP and every 6 months thereafter.
The Centers for Medicare & Medicaid Services (CMS) has recently issued a memo that authorized payment for CPAP may take place only if formal PSG was performed and was diagnostic for OSA, and that CMS will pay for CPAP therapy for 3 months (and subsequently if OSA improves) for adults diagnosed with either PSG or with unattended home sleep-monitoring devices. The use of portable home monitoring devices may improve access to diagnosis and treatment of OSA.
However, these devices must be used as part of a comprehensive sleep evaluation program that includes access to board-certified sleep specialists, PSG facilities, and therapists experienced in fitting and troubleshooting CPAP devices.
c. Oxygen therapy. Oxygen has been previously reviewed for the treatment of OSA, but the data are quite limited, including limited population. An AASM practice parameter review from 2006 did not recommend oxygen as a primary treatment for OSA. In contrast, in some cases, oxygen may be utilized as a supplement to positive airway pressure therapy (PAP) in cases of refractory hypoxemia, chronic obstructive pulmonary disease overlapping with sleep apnea, and may, in some circumstances, be an option for individuals who fail or refuse all other OSA treatments and have significant nocturnal hypoxia associated with their sleep apnea.
d. Oral appliances. Custom-made oral appliances improve upper airway size, and prevent the collapse of the tongue and soft tissues in the back of the throat by supporting the jaw in a forward position, keeping the airway open during sleep. One specific type of an oral appliance, the mandibular repositioning appliance, covers the upper and lower teeth and holds the mandible in a relatively advanced position with respect to the resting position, improving the air space. Oral appliances may not be as efficacious as CPAP in treating sleep apnea, but are indicated for use in patients with mild-to-moderate OSA who do tolerate or respond to CPAP, or fail behavioral interventions to improve compliance. Oral appliances are appropriate for first-line therapy in patients with primary snoring who do not respond to weight-loss or positional therapy (refers to avoiding sleep in the supine position, which tends to worsens OSA). Patients with CSA, morbid obesity, poor dentition, and acute TMD derangement are not good candidates for oral appliances.
e. Night shift. A special device that is worn on the back of the neck, and vibrates when patients sleep on their back and slowly increases in intensity until a position change occurs. The therapy may be effective for treatment of positional OSA alone or in combination with other sleep apnea treatments such as CPAP.
f. Winx sleep therapy system. The device works by generating negative pressure in the oral cavity, which draws the soft palate and uvula forward, and stabilizes the tongue position, thus enlarging the upper airway.
g. Expiratory positive airway pressure (or Provent Sleep Apnea Therapy). During inhalation, the device opens, allowing patients to breathe in freely. However, during exhaling, the valve closes, increasing the pressure in the airway, allowing patients to keep the airways open until the next inhalation cycle.
h. Somnoplasty. Also known as temperature-controlled radio frequency, is a minimally invasive surgical technique that utilizes radiofrequency current to reduce tissue volume in a precise, targeted manner. Data demonstrate that the technique reduces snoring but is ineffective for sleep apnea.
i. Uvulopalatopharyngoplasty (UPPP) with or without tonsillectomy.
This technique enlarges the retropalatal upper airway by excising a portion of the posterior soft palate and uvula. Anatomy is the main predictor of success. While favorable anatomy for UPPP includes large tonsils and favorable tongue placement (small base of tongue), the technique may not resolve sleep apnea or protect patients from future development of OSA. It is estimated that these surgical approaches are effective for approximately 50% of the time for amelioration of sleep apnea but are more effective for snoring. Thus, patients may continue to have silent obstructive apnea after surgery.
A 2010 AASM’s practice parameter on surgical treatment options for adult OSA patients reviewed the literature regarding the following specific surgical procedures: tracheostomy, maxillomandibular advancement, laser-assisted uvulopalatoplasty (LAUP), UPPP, RFA, and palatal implants.
Establishing a diagnosis of OSA and its severity by polysomnography prior to any surgical intervention was considered a standard recommendation by this position paper. In addition, in order that patients can make an informed decision regarding therapy, the standard proposed that all patients be advised of the anticipated success rates and potential complications, related to surgical intervention as compared to the alternative treatment options for their OSA (namely CPAP and oral appliances). If patients chose to have surgery, clinical follow-up including a nocturnal polysomnogram is a standard recommendation in order to demonstrate resolution of OSA as measures by the AHI, oxygen saturation, and sleep architecture. As for the specific surgical procedure, none, with the exception of LAUP, received more than a recommendation of option as an intervention for the management of OSA. The standard recommendation was not in favor of using LAUP as a treatment for OSA. A multidisciplinary approach is recommended to identify appropriate patients for surgical interventions.
j. Bariatric surgery. Gastric banding, sleeve gastrectomy, and gastric bypass surgery contribute to significant weight loss, resulting in improvements in OSA.
k. Inspire upper airway stimulation. An implanted system that senses breathing patterns and delivers mild stimulation to key airway muscles, by stimulating the hypoglossal nerve, which keeps the airway open during sleep.
l. Drug therapy. When nasal CPAP is not an option, patients with mild-to-moderate OSA may benefit from drug therapy. Protriptyline at a dosage of 10 mg at bedtime with upward adjustment depending on response and side effects may be an alternative treatment. Drug therapy is generally unsatisfactory for management of OSA. Recently, the FDA has approved the use of modafinil to improve wakefulness in patients who present with EDS associated with OSA if CPAP is used with adequate compliance and when total sleep time is adequate.
Key Points
• Sleep disturbances are very common in patients with neurologic disorders and may result in significant morbidity.
• Neurologists need to be aware of the major sleep disorders that may occur in their patients including chronic insomnia, sleep-disordered breathing such as obstructive sleep apnea, CNS hypersomnias including narcolepsy, circadian rhythm sleep disturbances, parasomnias such as RBD, and sleep-related movement disorders including RLS.
• Diagnosis is based on meticulous inventory of the clinical history, evaluation of the patients for problems such as snoring, apneic episodes, dream enactment, hypersomnolence, and urge to move the legs and an inability to initiate or maintain sleep.
• A detailed inventory of sleep patterns is an important step in the clinical interview, especially regarding bedtime habits and rituals that precede the typical sleep period and wake time, and daytime function.
• Questions reviewing the current medical and psychiatric problems, medications (with particular attention to activating and sedating drugs), social history, focusing on caffeine and alcohol ingestion as well as a family history for other first-degree relatives with similar complaints are very helpful.
• In some cases referral to a sleep laboratory for further evaluation with a nocturnal polysomnography (a sleep study) is indicated and may require the addition of additional EEG and EMG channels to evaluate for nocturnal seizures and parasomnias.
• RBD is increasingly recognized as a marker of an evolving neurodegenerative disease. It is very frequent in neurology practice, affecting up to two-thirds of patients with Parkinson’s disease and may be confused with nocturnal seizures and parasomnia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree