Fig. 16.1
(a, b) Microscopic appearances of ampullary somatostatinoma. Haematoxylin and eosin staining (left) and strongly positive immunostaining with somatostatin (right) (ANNALS IMAGE)
Psammoma bodies (round collections of calcium that arise after infarction and calcification) may be present in somatostatinomas. They are circular in appearance and are laminar, acellular and basophilic on histological examination. Psammomas are more commonly found in duodenal lesions and may be associated with neurofibromatosis [13].
16.5.3 Differentiation
Tumour differentiation – the extent of resemblance to normal cellular architecture – plays a role in assessing how aggressive a NET is [35]. Most somatostatinomas are well differentiated and have a uniform cellular pattern consistent with the organ of origin. Cells produce abundant neurosecretory granules and may also be arranged in nesting, trabecular or gyriform patterns [13, 35]. Poorly differentiated somatostatinomas less closely resemble non-neoplastic cells and have a more sheetlike or diffuse structural arrangement, with irregular nuclei and less cytoplasmic granularity.
16.5.4 Immunohistochemistry
Most NETs reveal some positivity for somatostatin immunohistochemistry due to the presence of somatostatin receptors. Somatostatinomas show diffuse positive immunoreactivity for somatostatin, and this expression is characteristic (Fig. 16.1) [3, 6, 13]. The immunoexpression is related to the differentiation of the tumour, with well-differentiated somatostatinomas revealing strong immunoreactivity for somatostatin staining and poorly differentiated tumours showing less immunoreactivity [35]. The absence of staining for other NET hormones (vasoactive intestinal polypeptide (VIP), gastrin, insulin and glucagon) also confirms the diagnosis. In addition, there may be immunoexpression of general neuroendocrine markers such as chromogranin A and synaptophysin [35].
16.5.5 Grading
Although no grading system can fully predict the behaviour of somatostatinoma, an estimate of the biological aggressiveness of NET can provide significant information on prognosis [35]. Biological activity can be assessed by cellular mitotic activity and the Ki-67 index (to estimate the growth fraction of a cellular population). Both of these measures assess tumour proliferative rate; mitotic activity requires counting the number of mitoses seen over a microscopic field, whereas the Ki-67 index provides a percentage of cells reacting to labelling with MIB1 antibody [35, 36]. These measures are broadly equivalent in assessing grading but can sometimes give conflicting information (in which case the more aggressive score is adopted) [35]. If limited amounts of tissue are available (e.g. following biopsy), a mitotic index may not be possible to perform as it requires counting 40–50 high-power microscopic fields (more than most biopsy samples contain). In these cases, Ki67 staining provides a more accurate assessment of proliferative rate [35].
Histological grading for pancreatoduodenal NETs is either low (G1), intermediate (G2) or high (G3) (Table 16.1). Low-grade NETs are relatively indolent, while intermediate grade tumours have a less predictable, moderately aggressive course [35]. Somatostatinomas that are either G1 or G2 tend to be well-differentiated NETs, while G3 tumours are poorly differentiated neuroendocrine tumours [35, 36].
16.6 Staging
A number of different staging systems exist to classify the extent of tumour spread for NETs, and while the criteria for assessment vary between each method, the underlying basic data are similar [35]. The TNM staging system is becoming the most widespread method for assessment; it reveals the extent of invasion into the organ of origin and regional or distant spread (Table 16.2) [35, 36].
Table 16.2
TNM classification for pancreatoduodenal neuroendocrine tumours
TNMgrade | Duodenum/ampulla/proximal jejunum | Pancreas |
|---|---|---|
Tx | Primary tumour cannot be assessed | Primary tumour cannot be assessed |
T0 | No evidence of primary tumour | In situ tumour/dysplasia |
T1 | Tumour invades lamina propria or submucosa and size ≤1 cm | Tumour invades lamina propria or submucosa for ≤1 cm |
T2 | Tumour invades muscularis propria or size ≥1 cm | Tumour invades muscularis propria or subserosa for ≥1 cm |
T3 | Tumour invades the pancreasof the retroperitoneum | Tumour penetrates serosa |
T4 | Tumour invades peritoneum or other organs | Tumour invades adjacent structures |
Nx | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis | No regional lymph node metastasis |
N1 | Regional lymph node metastasis | Regional lymph node metastasis present |
Mx | Distant metastases cannot be assessed | Distant metastases cannot be assessed |
M0 | No distant metastases | No distant metastases |
M1 | Distant metastases | Distant metastases |
16.7 Presentation
The functionality of the somatostatinoma greatly influences presentation. Non-functional tumours, which are not associated with somatostatinoma syndrome, tend to present with local mass effects, though many are detected incidentally. Symptoms are non-specific but include upper abdominal pain, abdominal swelling and mass, jaundice, weight loss, nausea and vomiting [3]. Some patients will present with tumour burden from metastatic disease. Often non-functioning tumours are detected incidentally during investigation of non-specific gastrointestinal symptoms in the same manner as other gastroduodenal NETs.
16.7.1 Somatostatinoma Syndrome
Functioning tumours are rare and cause somatostatinoma syndrome as a result of overexpression of somatostatin. This syndrome comprises diarrhoea (secondary to decreased pancreatic enzyme and bicarbonate secretion), steatorrhoea and diabetes (resulting from insulin inhibition), gallstones (from cholecystokinin inhibition) and hypochlorhydria [4–6]. Some authors argue that this syndrome must be present for a secure diagnosis of somatostatinoma [7]. Functioning tumours are likely to present earlier than non-functioning tumours, given their symptomatology and consequent investigation.
16.8 Investigation
Although radiology provides the mainstay of investigation, it will only reveal the presence of a pancreatoduodenal NET rather than a definitive diagnosis of a somatostatinoma [3]. Nuclear medicine and endoscopic assessment play an increasingly important role in the management of this group of tumours. Biochemical assessment may also reveal the presence of a NET, and it can be diagnostic for somatostatinoma. Definitive diagnosis tends to come from tissue diagnosis and immunohistochemistry (either biopsy or pathological specimen).
16.8.1 Biochemical and Haematological Assessment
Patients with pancreatoduodenal NETs should be investigated with both standard and specific blood tests. Routine full blood count, liver function tests, urea and electrolytes and clotting screen should be performed to check for any derangement in organ function: significant hepatic metastases may result in derangement in liver function. Blood glucose should also be measured for signs of diabetes, either due to somatostatin syndrome or to pancreatic dysfunction secondary to tumour invasion. These tests should also be used as a workup for further management, both in terms of radiological investigation and suitability for operative intervention.
Chromogranin A and pancreatic polypeptide are non-specific markers for pancreatoduodenal NETs; elevated levels are found in 50–80 % of tumours, including somatostatinomas [20]. Although elevated plasma somatostatin levels (SLI) are strongly indicative of somatostatinoma, they are rarely found [5, 7]. Pancreatic somatostatinomas tend to have higher SLI concentrations (up to 50 times normal) compared to intestinal somatostatinomas in which the concentration is often normal [6]. Patients with high plasma levels of somatostatin are thought to present earlier than those with normal levels [4].
16.8.2 Radiological Imaging
Accurate imaging of the primary tumour and the extent of disease is vital in all stages of management of somatostatinomas. Imaging should provide the location and extent of the primary tumour and assist in determining whether operative intervention should be performed, either curative resection or debulking. In addition, local invasion and the presence of metastases should be sought on investigation. Functional somatostatinomas may be harder to identify than tumours without somatostatinoma syndrome, as they are likely to be smaller at initial presentation.
Conventional imaging provides the mainstay of initial investigation. Computed tomography (CT), ultrasound scanning and magnetic resonance imaging (MRI) can all be employed to assist diagnosis, with selective use of small bowel series (barium or Gastrografin) (Fig. 16.2) and angiography (Fig. 16.3) [3]. Dual-phase, thin-slice CT (Fig. 16.4) is the usual first-line investigation; detection rates are proportional to the size of the lesion. Somatostatinomas are isodense (and thus not visible on unenhanced CT), but intravenous contrast will reveal a characteristic hypervascular lesion [38]. Typically lesions are 5 cm in diameter at presentation [34]. More than 70 % of lesions greater than 3 cm in size can be identified, but only 50 % of lesions measuring less than 1 cm are detected [10, 39]. Thus, small somatostatinomas and hepatic metastases may be missed. Dual-phase CT and MRI have equivalent sensitivities for the detection of NETs; CT is thought to be better for detection of peritoneal and mesenteric disease than MRI, which is more sensitive for detecting liver and bony metastases [6, 40].
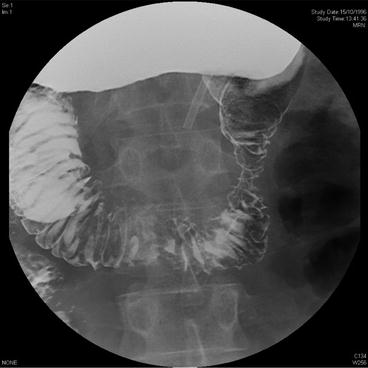
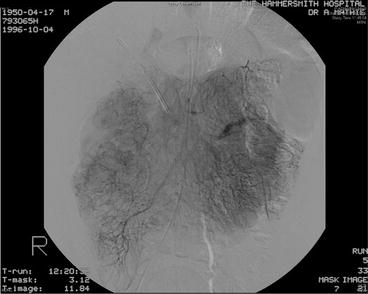
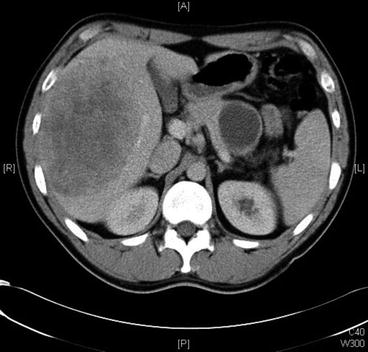

Fig. 16.2
Small bowel Gastrografin study showing constricting lesion in the second part of the duodenum (patient in prone position). Lesion subsequently confirmed as somatostatinoma on postoperative immunohistochemistry

Fig. 16.3
Visceral angiography of patient with duodenal somatostatinoma. Increased tumour ‘blush’ noted at the right-hand side of the image, but no gross vascular invasion present (patient subsequently underwent successful pylorus-preserving proximal pancreatoduodenectomy)

Fig. 16.4
Computed tomography showing the presence of a large pancreatic primary somatostatin and hepatic metastases
Transabdominal ultrasound is not used routinely because it has a low sensitivity (9–64 %) for pancreatic lesions [10, 39]. Angiography may reveal a characteristic tumour blush and can be combined with selective visceral cannulation and assessment of hormonal gradients. These techniques have now been largely superseded by other imaging modalities, notably CT.
16.8.3 Nuclear Medicine Imaging
16.8.3.1 Somatostatin Receptor Scintigraphy
The overexpression of somatostatin receptors (particularly subtypes SSTR2 and SSTR5) by more than 80 % of pancreatoduodenal NETs has allowed the development of somatostatin receptor scintigraphy (SRS) [31, 41, 42]. A number of different radiolabelled somatostatin analogues will bind to the receptors with high affinity. One of the agents most widely used worldwide for SRS is 111IN-DTPA-octreotide (Octreoscan), which has an overall sensitivity of 80–90 % [6, 10, 20, 31, 43]. SRS allows whole body scanning, which is invaluable in detecting metastases (especially at unexpected locations), but it does not provide information on tumour size or resectability [4, 6]. After the radiolabelled agent is injected into the patient, scans are generally performed at either 4–6 h or at 24 h. With earlier scans, lesions may be obscured by a relatively high background activity. Delayed scans provide better contrast (due to lower background activity) but can result in false-positive results secondary to physiological bowel activity [6].
16.8.3.2 SPECT (Somatostatin Receptor Scintigraphy Combined with Computed Tomography)
SPECT is more sensitive than conventional imaging for detection of both the primary somatostatinoma and its metastases [31, 44]. Seventy per cent of primary lesions and more than 90 % of distant disease can be detected with SPECT [31, 44]. It has the additional advantage over conventional imaging that it can provide whole body scanning at one time, and it alters patient management in up to 47 % of patients [7, 31]. False-positive localisations can occur in up to 12 % of patients, but this figure can be reduced to 3 % when findings are corroborated with clinical assessment [7, 10, 31].
16.8.3.3 Positron Emission Tomography
Positron emission tomography scanning (Fig. 16.5) has greater sensitivity than either SRS or conventional cross-sectional imaging [10]. As a result, this new technique is becoming increasingly employed for the detection of all pancreatoduodenal NETs, including somatostatinomas [3]. Two main agents are used: 11C-5-HTP and 68gallium-labelled somatostatin analogues.
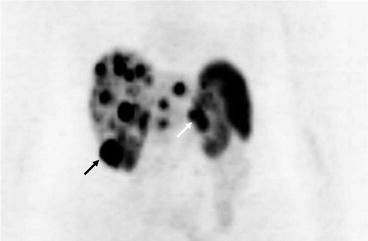

Fig. 16.5
PET scan showing extensive hepatic metastases as well as uptake in the inferior pole of the right kidney (black arrow) and the pancreatic primary tumour (white arrow)
16.8.4 Endoscopic Examination
Endoscopy allows direct visualisation of the upper gastrointestinal tract and can help diagnose gastroduodenal lesions. It should be combined with concurrent endoscopic ultrasound, which can detect lesions as small as 0.5 cm [45]. Endoscopic ultrasound is more effective at localising tumours in the pancreas than the duodenum, although the tail of the gland can be inaccessible [7, 46]. Fine needle aspirates can be taken, which can be useful in providing cytological assessment of suspect lesions.
16.8.5 Intraoperative Techniques
Both intraoperative ultrasonography and endoscopic transillumination are recommended to assist in detection of small lesions not appreciated on conventional imaging [7, 10]. In addition they can be used to help plan the route of resection and enucleation of lesions [4]. However, these techniques may fall out of routine use with the increasing availability and sensitivity of nuclear imaging.
16.9 Management
The overall 5-year survival rate in pancreatic and periampullary somatostatinoma is 60–100 % with localised disease or 15–60 % in metastatic disease [15, 47]. Large size (>3 cm), poor differentiation and lymph node involvement are poor prognostic markers. Tumours that are hormonally inactive (non-functioning), which predominate, have a worse prognosis than functioning somatostatinomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






