The operator-independent “hands-free” ultrasound device for transcranial insonation of proximal intracranial arteries through standard temporal and suboccipital windows.
Focused questions
Does the stroke center have personnel trained in TCD ultrasound monitoring, which includes insonating and identifying intracranial arteries and interpretating findings associated with acute thromboembolic occlusions?
Does the stroke center have the resources to provide TCD ultrasound monitoring twenty-four/seven?
Would a certifying body be a prerequisite for center/physician to ensure accepted and standard performance of the procedure?
Anatomy and acoustic windows for sonothrombolysis
For the purposes of sonothrombolysis, acoustic energy can be delivered through three acoustic windows on the skull to cover the proximal intracranial arteries where acute thromboembolic occlusions commonly occur (Figure 14.2). Briefly, the standard TCD temporal windows are used to insonate the M1-proximal M2 middle cerebral artery, terminal internal carotid, A1 anterior cerebral and P1-proximal P2 posterior cerebral arteries, while the suboccipital window is used to insonate the terminal vertebral and basilar arteries.
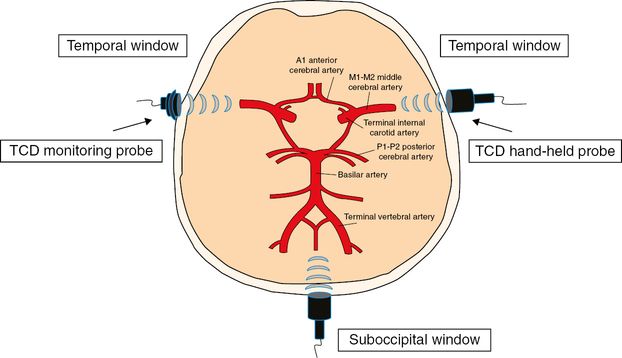
Schematic illustration of continuous TCD monitoring of the circle of Willis via standard acoustic bone windows. TCD = transcranial Doppler.
Procedure tips
Any commercially available TCD system that emits high-frequency 2-MHz ultrasound through a hand-held probe can be utilized for sonothrombolysis (these devices are designed not to exceed the Food and Drug Administration-mandated ultrasound intensity limits for diagnostic ultrasound systems – derated spatial peak temporal average intensity <720 mW/cm2). An adjustable headframe, which is commercially available, should be used to ensure continuous and steady exposure to ultrasound at the site of the occlusion.
Steps of sonothrombolysis
Patients satisfying the inclusion and exclusion criteria for tPA administration will receive the recommended dose of intravenous tPA (0.9 mg/kg body, 10% given by bolus and rest of the 90% to be given by infusion for 1 hour).
To avoid significant time delay between initiation of intravenous thrombolysis and continuous ultrasound exposure, TCD examination should begin with the maximum power and gate settings (i.e., power 100%, gate 15 mm) to promptly find the acoustic window and identify the different intracranial arterial segments by using a standard fast-track insonation protocol [24]. If power motion Doppler is used, a smaller gate (e.g., 3mm) can be chosen instead.
Find the worst residual flow identified as the lowest Thrombolysis in Brain Ischemia (TIBI) flow grade [25] or lesions amenable to intervention [26] in the affected vessel and store it digitally as the baseline signal (Figure 14.3 and Table 14.1).
Once the lowest TIBI flow grade has been identified and stored, the ultrasound-monitoring probe should be fixated with the headframe to avoid probe movements and related artifacts during sonothrombolytic treatment. Repeat a quick examination with the fixated (but adjustable) monitoring probe until the lowest TIBI signal is found and tighten the probe in the holder. Alternatively, if a proximal occlusion has been identified by any other vascular imaging modality (e.g., computed tomography angiography) prior to tPA bolus, identification of the worst TIBI flow grade can be accomplished through the monitoring probe in the first place to avoid delay in the initiation of TCD monitoring.
The contact between the probe and the skin should be firm to ensure maximum transmission of acoustic energy to the occlusion site.
Continuous TCD monitoring should be performed for 2 hours, while the received flow signals should be digitally stored every 30 minutes or in case of any improvement or worsening in the TIBI flow grades, respectively.
| Lesion location | TCD criteria (at least one present) |
|---|---|
| M1-/M2-MCA | Primary: TIBI flow grades 0–4 (absent, minimal, blunted, dampened or stenotic) at depths <45 mm (M2) and 45–65 mm (M1) Secondary: flow diversion to ACA, PCA or M2-MCA. Increased resistance in unilateral terminal ICA. Embolic signals in MCA. Turbulence, disturbed flow at stenosis. Nonharmonic and harmonic co-vibrations (bruit or pure musical tones) |
| Distal ICA | Primary: TIBI flow grades 0–4 at 60–70 mm. Increased velocities suggest anterior cross-filling of collateral flow through anterior or posterior communicating arteries. Reversed unilateral OA. Delayed systolic flow acceleration or blunted ipsilateral MCA, MFV >20 cm/s Secondary: Embolic signals in the unilateral MCA. Orthograde (or retrograde) flow in unilateral OA due to retrograde filling of siphon |
| Tandem ICA/MCA | Primary: TIBI grade 0–4 and increased velocities in contralateral ACA, MCA or unilateral posterior communicating artery or reversed unilateral OA Secondary: delayed systolic flow acceleration in proximal MCA or terminal ICA. Embolic signals in proximal MCA or terminal ICA |
| Basilar artery | Primary: TIBI flow grade 0–4 at 75–100 mm Secondary: flow velocity increase in terminal VA and branches, MCA or posterior communicating arteries. High-resistance flow signals in VA(s). Reversed flow direction in distal BA |
| Vertebral artery | Primary (intracranial VA occlusion): TIBI flow grades 0–4 at 40–75 mm Primary (extracranial VA occlusion): absent, minimal, or reversed high resistance flow signals in unilateral terminal VA Secondary: embolic signals. Increased velocities or low pulsatility in contralateral VA |
Future directions
To further augment the effect of intravenous tPA on clot lysis, the addition of gaseous microbubbles to continuous ultrasound monitoring seems to be a potentially beneficial approach in treatment of acute ischemic stroke [27]. Several smaller monocentric studies utilizing 2-MHz sonothrombolysis in combination with galactose- or lipid-based microbubbles showed promising recanalization rates and signal-of-efficacy with regard to short- and long-term outcomes in patients with acute large vessel occlusions [28–30].
In light of the above-mentioned evidence for the efficacy of endovascular therapy, the question arises whether bridging with sonothrombolysis followed by endovascular reperfusion therapy further enhances recanalization rate and functional outcome in patients with large vessel occlusion. Moreover, while the majority of stroke-treating hospitals still lack neuroendovascular resources, sonothrombolysis utilizing the currently tested operator-independent ultrasound device could be implemented in a “drip, insonate and ship” approach with intravenous tPA and transcranial ultrasound initiated by the referring hospital and continued during patient transport to the closest neuroendovascular center.
Future randomized controlled trials in large study populations are needed to investigate efficacy of these promising approaches and demonstrate generalizability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









