Spinal Cord
Anatomy of the Spinal Cord
Gross Anatomy and Relationship to Vertebral Levels
Anchored to the dura by the dentate (denticulate) ligaments, the spinal cord [15,18,22,29,36] extends from the level of the cranial border of the atlas, where it is continuous with the medulla, to the lower border of the first lumbar vertebra. During early fetal development, the spinal cord extends to the lower end of the sacrum, but at birth it extends only as far as the upper border of the third lumbar vertebra. At 20 weeks’ gestational age, the conus medullaris is at the L4–L5 level. By 40 weeks’ gestational age or at term, the conus medullaris is at the L3 level. By age of 2 months, it has reached the adult L1–L2 level [6]. The average length of the spinal cord is 45 cm in the adult male and 42 to 43 cm in the adult female. The corresponding average length of the spinal column is 70 cm. Cylindrical and flattened in a dorsoventral direction, the spinal cord fills one-third to one-half of the vertebral canal, and demonstrates both cervical and lumbar enlargements. The first enlargement corresponds to the segmental innervation of the upper extremities and extends from the C5 to T1 spinal levels, whereas the second corresponds to the innervation of the lower extremities and extends from L3 to S2. Below the lumbar enlargement, the spinal cord narrows, ending as the conus medullaris. From the conus medullaris, a fine pial thread known as the filum terminale passes down to the dorsum of the first coccygeal segment.
Although the spinal cord is a continuous and nonsegmental structure, the 31 pairs of nerves originating from it give it a segmental appearance. On this basis, the spinal cord is considered to have 31 segments analogous to the spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal). Due to the different growth rates of the spinal cord and the vertebral column, the more caudal (lumbar and sacral) spinal roots must travel a considerable distance in the subarachnoid space before they reach their corresponding intervertebral foramina. This lower group of roots congregates around the filum terminale in the spinal theca and is known as the cauda equina.
At the cervical level, the relation between the spine and the spinal cord roughly corresponds. However, the thoracic spinal cord is located in the spinal canal formed by the first to the eighth (T1–T8) thoracic vertebrae, the lumbar spinal cord in the canal formed between the ninth and the eleventh (T9–T11) thoracic vertebrae, and the sacral spinal cord in the canal formed between the twelfth thoracic (T12) and the second lumbar (L2) vertebrae. In the cervical region, the spinous process of a particular vertebra matches the level of the corresponding cord segment; in the upper thoracic region, there is a discrepancy of two segments (e.g., the fourth thoracic spinal segment overlies the sixth) and in the lower thoracic region, there is a discrepancy of three segments. The eleventh thoracic spinous process overlies the third lumbar cord segment, and the twelfth overlies the first sacral cord segment.
The external surface of the spinal cord is marked by a ventral median fissure and a dorsal median sulcus (continued by a dorsal median septum), which divide the cord into two symmetric halves. In the posterolateral surface, there is a dorsolateral sulcus marking the entrance of the dorsal roots. In the anterolateral surface, there is a ventrolateral sulcus that is not as well delineated as the other sulci because the ventral roots emerge as a number of separate twigs.
Cross-sectional Anatomy of the Spinal Cord
In its cross-section (Fig. 5.1), the spinal cord consists of the centrally placed gray matter surrounded by white matter. The gray matter is shaped like a modified H, with two lateral columns joined by a transverse commissure. Each lateral column has a dorsal horn lying dorsolaterally and a ventral horn lying ventrolaterally. The central canal of the spinal cord is located in the center of the gray commissure.
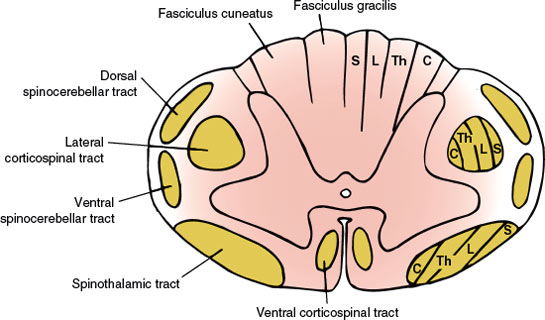
FIG. 5.1. Anatomy of the spinal cord (cross section). Tract lamination: S = sacral segments; L = lumbar segments; Th = thoracic segments; C = cervical segments.
In addition to these gray columns, there is an intermediolateral gray column that extends from segments T1 through L2 and gives rise to preganglionic sympathetic autonomic fibers. There is also an intermediolateral zone of gray matter in the second, third, and fourth sacral segments, which is the source of the sacral portion of the parasympathetic outflow. A laminar architecture of nine cell layers or laminae is distinguishable within the gray matter: laminae I through VI (dorsal horn), lamina VII (intermediate zone), and laminae VIII and IX (ventral horn). The zone of Lissauer (posterolateral tract of Lissauer) separates the dorsal gray column from the surface of the spinal cord. The portion of the gray matter dorsal to the central canal is the dorsal gray commissure and that of the ventral portion is the ventral gray commissure.
LAMINA
| I | Nucleus posteromarginalis |
| II | Substantia gelatinosa |
| III | and IV Nucleus proprius dorsalis |
| V | Zone anterior to lamina IV |
| VI | Zone at the base of dorsal horn |
| VII | Intermediate zone |
| VIII | Zone in the ventral horn (restricted to medial aspect in cervical and lumbar enlargements) |
| IX | Medial and lateral anterior horn cell columns |
Each half of the white matter of the spinal cord is separated into three funiculi by the gray matter and the intramedullary portions of the spinal roots, as follows:
1. The dorsal funiculus: the portion of white matter between the dorsomedian and the dorsolateral sulci
2. The lateral funiculus: the white matter between the dorsolateral and the ventrolateral sulci
3. The ventral funiculus: the white matter between the ventrolateral sulcus and the ventromedian fissure
Bands of white matter known as the dorsal and ventral white commissures correlate with the gray commissure. The white matter comprises ascending and descending tracts.
Major Ascending and Descending Tracts of the Spinal Cord
ASCENDING TRACTS
Almost all the sensory afferent input to the spinal cord enters by way of the dorsal roots. The central end of a dorsal root splits into lateral and medial bundles. The finely myelinated or unmyelinated fibers of the lateral bundle bifurcate into short ascending and descending branches within the zone of Lissauer and terminate on the neurons of the dorsal horn. The axons of second-order neurons, with cell bodies presumably in laminae VI and VII, decussate over several segments by way of the ventral white commissure; they proceed to the ventrolateral quadrant of the spinal cord and ascend as the lateral spinothalamic (neospinothalamic) tract to reach the thalamus (ventral posterolateral nucleus). These fibers convey pain and temperature sensation and have a laminar configuration. As a consequence of this arrangement, fibers carrying information from cervical regions lie dorsomedially and those from sacral regions lie ventrolaterally. There also seems to be a segregation between pain and temperature fibers, with fibers carrying temperature information located dorsolaterally to pain-carrying ones.
Pain may also be conducted by way of the spinoreticulothalamic (paleospinothalamic) system. Fibers from this system have short axons that synapse in the brainstem reticular formation and terminate in the intralaminar nuclei of the thalamus. The lateral spinothalamic tract conveys information that is perceived as sharp and localized pain, whereas the spinoreticulothalamic system is concerned with poorly localized pain sensation.
Fibers carrying tactile sensibility (light touch) also bifurcate after entering the zone of Lissauer and terminate with interneurons of the dorsal horn. The axons of second-order neurons, whose cell bodies presumably lie in laminae VI and VII, crossover to the opposite side through the ventral white commissure and ascend as the ventral spinothalamic tract to reach the ventral posterolateral nucleus of the thalamus. Light touch is also transmitted by way of the dorsal funiculus–medial lemniscus pathway.
The heavily myelinated fibers of the medial bundle of the dorsal root pass over the dorsal horn into the dorsal funiculus. After giving rise to collaterals, which terminate largely in laminae III and IV, they ascend in the dorsal funiculus. The fibers from the lowermost part of the body (sacral, lumbar, and lower six thoracic levels) are located more medially and constitute the fasciculus gracilis, whereas those coming from the upper part of the body (upper six thoracic and all cervical levels) occupy a more lateral position and constitute the fasciculus cuneatus. The fasciculus gracilis ends in the nucleus gracilis of the medulla; the fasciculus cuneatus also reaches the dorsal surface of the medulla and terminates in the nucleus cuneatus. The axons of these two nuclei decussate in the lower medulla and ascend as the medial lemniscus to reach the ventral posterolateral nucleus of the thalamus. These fibers carry information concerning discriminative senses (position sense, vibration sense, weight perception, discriminative touch, pressure touch, two-point discrimination, stereognosis, and shape and movement awareness). Other ascending tracts include the dorsal spinocerebellar and ventral spinocerebellar tracts, which transmit unconscious proprioceptive information from the lower limbs and the inferior half of the body to the cerebellum and the cuneocerebellar and rostrocerebellar tracts, which convey similar information from the upper limbs and rostral half of the body.
DESCENDING TRACTS
Five descending systems exert tonic effects on the a and γ motor neurons; these systems are therefore important in the postural control of the limbs. Two of these systems (the vestibulospinal tract and the medial reticulospinal tract) tend to facilitate the α and γ motor neurons of antigravity muscles and the other three systems (the corticospinal tract, the corticorubrospinal tract, and the lateral reticulospinal tract) inhibit the antigravity muscles and facilitate the antagonists.
Corticospinal Tract
The fibers of the corticospinal pathway arise mainly from somatotopically organized areas from the primary motor cortex, lateral premotor cortex, and supplementary motor cortex of the contralateral hemisphere. The corticospinal neurons are found primarily in Brodmann’s area 4, which occupies the posterior portion of the precentral gyrus (primary motor cortex or MI). The lateral premotor and supplementary motor cortices are located in Brodmann’s area 6. Corticospinal axons also arise from neurons in the primary sensory cortex in the postcentral gyrus (Brodmann’s areas 3, 1, and 2), anterior paracentral gyri, superior parietal lobule (Brodmann’s areas 5 and 7), and portions of the cingulate gyrus on the medial surface of the hemisphere. These fibers descend through the corona radiata, the posterior limb of the internal capsule, and the ventral portion of the mesencephalon and pons down to the ventral portion of the medulla, where they form two large pyramids. When they reach the caudal portion of the medulla, approximately 90% of the 1 million fibers of each pyramid cross over in an interdigitated fashion to descend in a massive tract in the lateral funiculus of the spinal cord known as the lateral corticospinal tract. The fibers in the lateral corticospinal tract extend all the way through the spinal cord and terminate in laminae IV through VII and IX. The other 10% of the fibers that do not decussate descend in the ipsilateral ventral funiculus as the ventral corticospinal tract, which terminates (after crossing in the ventral white commissure) in lamina VIII of the cervical and upper thoracic regions.
Motor neurons that innervate the axial musculature are situated in an extreme ventromedial sector of lamina IX, whereas those that innervate the intrinsic extremity musculature are clustered within a dorsolateral sector; motor neurons for the limb girdle musculature are located in an intermediate position [2]. This ventromedial–dorsolateral gradient of the proximal–distal representation is also maintained within the intermediate zone (laminae V–VIII), which contains the propriospinal neurons. Propriospinal neurons projecting to the limb and axial motor neurons tend to project for considerable distances above and below the segment of origin, thereby influencing large groups of proximal muscles. By contrast, propriospinal neurons projecting to motor neurons that innervate the intrinsic muscles of the limb tend to project only short distances above and below the segment of origin, thereby influencing smaller, more restricted groups of distal muscles [2].
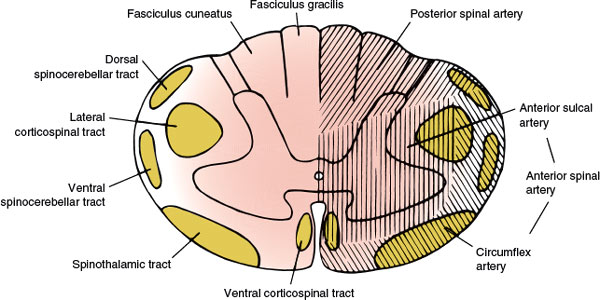
FIG. 5.2. The vascular supply of the spinal cord.
Corticorubrospinal Tract
Cells in cortical areas 4 and 6 and 1, 2, and 3 project to the ipsilateral red nucleus. The axons of some of these rubral cells decussate and descend through the brainstem tegmentum and lateral funiculus of the spinal cord as the rubrospinal tract.
Lateral Reticulospinal Tract
This tract originates in the medullary reticular formation, chiefly on the ipsilateral side, and descends in the ventrolateral funiculus.
Vestibulospinal Tract
Fibers originating in the lateral vestibular nucleus extend through the entire length of the spinal cord in the anterior region of the lateral funiculus. Fibers from the medial vestibular nucleus extend through the cervical and upper thoracic levels in the ventral funiculus.
Medial Reticulospinal Tract
This pathway originates in the pontine and lateral medullary reticular formations and descends largely uncrossed in the ventral funiculus of the spinal cord.
Arterial Supply to the Spinal Cord
The main arterial supply to the spinal cord arises from the anterior spinal artery (running along the anterior median fissure), the paired posterior (posterolateral) spinal arteries (coursing along the posterolateral sulcus), and the perimedullary plexus connecting them. The vascular supply of the spinal cord (Fig. 5.2) is divided into extraspinal and intraspinal systems [34,104,132].
EXTRASPINAL SYSTEM (EXTRAMEDULLARY ARTERIES)
The lateral spinal arteries, present in early development, give rise to radicular arteries, radiculopial arteries, and radiculomedullary arteries. The latter, by means of the anterior and posterior spinal arteries, are responsible for most of the blood supply to the spinal cord. The radiculomedullary arteries are present only at certain segmental levels. Approximately 6 to 10 of them join the anterior spinal artery, running along the anterior median fissure, and 10 to 23 join the posterior spinal arteries. The anterior spinal artery arises from the anastomosis of two branches from the vertebral artery, extends from the level of the olivary nucleus to the tip of the conus medullaris, and supplies the ventral surface of the medulla and the anterior two-thirds of the spinal cord. Sulcal branches of the anterior spinal artery supply the anterior horn, the lateral horn, the central gray matter, and the basal aspect of the posterior horn. The posterior spinal arteries are paired branches of the intracranial vertebral artery or the posterior inferior cerebellar artery. The posterior spinal arteries also extend the length of the cord and supply the posterior horn and the posterior funiculus. This arterial system receives 10 to 20 posterior radicular vessels [34,132]. At the conus medullaris, the anterior and posterior spinal arteries are joined by the anastomosing ansa of the conus. Anastomotic radicular arteries, most of them branches of the aorta, feed both the arterial systems at various levels.
Three main functional regions in the vertical axis of the spinal cord have been distinguished according to their unequal blood supply:
1. The upper or cervicothoracic region, richly vascularized, embraces the cervical and first two thoracic cord segments. The first four cervical segments are supplied by the anterior spinal artery and have limited or no radiculomedullary supply. The lower four cervical and the first two thoracic segments receive their supply from two to four large radicular arteries arising from the vertebral and the ascending and deep cervical arteries. The most important of these radicular arteries is named the artery of the cervical enlargement typically arising between C4 and C8; it usually enters the spinal cord with the seventh and eighth cervical roots. A variable number of radiculomedullary vessels feed the posterior spinal arteries, and they predominate in the cervical enlargement.
2. The intermediate or midthoracic region, poorly vascularized, is supplied by the branches of the intercostal arteries and includes the third through the eighth thoracic segments. It receives a single radiculomedullary artery, which enters with the sixth, seventh, or eighth thoracic roots. There are two to three segmental feeders to the posterior spinal arteries.
3. The lower or thoracolumbosacral region enjoys a rich vascularization and is nourished by radiculomedullary branches of the intercostal and lumbar arteries. The most important source of supply to the anterior circulation depends on the great anterior radicular artery of Adamkiewicz (artery of the lumbar enlargement) that enters most frequently from the left side with the ninth, tenth, eleventh, or twelfth thoracic (75% of cases between T9 and T12) or first two lumbar roots, seldom from the lumbar region or higher segments between T6 and T8. Numerous posterior radicular arteries are also present in this region.
INTRASPINAL SYSTEM (INTRAMEDULLARY ARTERIES)
Branches of the anterior and posterior spinal arteries form a perimedullary circuitry around the cord. Branches arise from this plexus to supply a substantial amount of white matter and the dorsal horns of the gray matter. The arterial supply of the gray matter is richer than that of the white matter. The largest branches of the anterior spinal artery (sulcocommissural arteries) enter the ventral median fissure and supply the gray matter, except for the dorsal horns and the innermost portion of the white matter. The dorsal horns and funiculi are supplied by the paired posterior spinal arteries, the posterior medullary feeders, and the perforating pial branches.
Venous Drainage of the Spinal Cord
Veins draining the spinal cord have a distribution similar to arteries. Anterior longitudinal trunks consist of anteromedial and anterolateral veins. Sulcal veins drain the anteromedial portion of the spinal cord. Anterolateral regions of the spinal cord drain into the anterolateral veins. Posterior longitudinal venous trunks drain the dorsal funiculus. Throughout the coronal venous plexus, they are interconnected. At each spinal cord segment small radicular veins drain the nerve roots, but at some levels larger medullary veins will arise from the anterior median spinal vein. There are approximately 10 to 20 anterior medullary veins and a similar number of posterior medullary veins. The posterior half of the spinal cord drain into the posterior, and the anterior half of the spinal cord drain into the anterior medullary veins. These medullary veins unite with the radicular veins, internal and external vertebral plexus to form the intervertebral vein that drains blood from the spine and spinal cord. Prior to their exit from the dura mater, these veins are valveless.
Within the spinal canal’s epidural space (bounded by the posterior longitudinal ligament anteriorly, the ligamenta flava and the periosteum of the laminae posteriorly, and the pedicles of the spinal column and the intervertebral foramina laterally), there is also a longitudinally and circumferentially arranged network of valveless veins, known as the internal venous plexus (anterior and posterior). The internal venous plexus, lying external to the dura, communicates with the spinal cord through the medullary and radicular veins and with the vertebral body through basivertebral veins that run horizontally within the vertebrae, and drains into the external venous plexus (anterior and posterior), which surrounds the vertebral column. Both the internal and external venous plexus extend from the base of the skull to the sacrum. The vertebral venous plexuses also anastamose with the sacral, pelvic, and prostatic venous plexuses [95]. The vertebral venous plexus is often referred to as Batson’s plexus. These vascular networks of valveless veins, allowing bidirectional flow, provide a direct vascular route for the spread of prostate and other pelvic tumors.
Physiology of the Spinal Cord Circulation
Spinal cord blood vessels autoregulate in response to changes in systemic arterial blood pressure; they dilate when the PaCO2 is increased, and constrict when it is reduced. Spinal cord perfusion is more directly affected by changes in systemic blood pressure than brain perfusion. Low peripheral vascular resistance with aortic hypotension diverts aortic outflow away from the spinal cord blood vessels. When peripheral vascular resistance in the lower extremities is high, aortic outflow is diverted toward the spinal circulation, increasing pressure and possibly spinal cord perfusion. If patient has hypertension and increased peripheral vascular resistance, a column of contrast medium in the aorta during aortography is diverted into the spinal cord circulation. If peripheral resistance is decreased, rapid forward flow occurs, and the aorta is virtually emptied of contrast at the end of one cardiac cycle and none is visible in the spinal arterial circulation.
Covered by the three membranes of the CNS, the dura mater, arachnoid, and the innermost piamater, the spinal cord is encased within a bony structure similar to that which protects the brain, so that any change in volume can take place only at the expense of the cerebrospinal fluid (CSF), the blood, or the spinal cord tissue itself. Damage to the spinal cord typically occurs with more than 20 to 30 minutes of ischemia and during systemic hypotension with failure of the autoregulatory response. The resulting ischemic injury to the spinal cord affects areas with high metabolic demand, such as the anterior horn cells and gray matter before, leading to complete cord necrosis [104].
Lesions of the Spinal Cord
Spinal cord syndromes may develop acutely (within minutes or hours), subacutely (days or weeks), or chronically (developing over months or even years). According to the degree of functional impairment, spinal cord syndromes may be further classified as complete or incomplete.
Complete Spinal Cord Transection (Transverse Myelopathy)
With complete cord transection (Fig. 5.3), all ascending tracts from below the level of the lesion and all descending tracts from above the level of the lesion are interrupted [6,30,43]. Therefore, all motor and sensory functions below the level of spinal cord damage are disturbed. More often, the section is incomplete and irregular, and the findings reflect the extent of the damage. Transverse myelopathy of acute onset is often due to traumatic spine injuries, tumor (e.g., metastatic carcinoma, lymphoma), multiple sclerosis, or vascular disorders. Other causes include spinal epidural hematoma or abscess, paraneoplastic myelopathy, autoimmune disorders, herniated intervertebral disc, and parainfectious or postvaccinal syndromes [59]. The main causes of transverse myelopathy are summarized in Table 5.1.
TABLE 5.1 Transverse Myelopathy

SENSORY DISTURBANCES
All sensory modalities (soft touch, position sense, vibration, temperature, and pain) are impaired below the level of the lesion. Clinically, pinprick loss below a segmental level is most valuable in localizing the lesion. A sensory level may be easily missed unless carefully, and sometimes repeatedly, sought. In complete lesions, particularly with extramedullary pathology, the sensory level may be many segments below the level of the lesion. For instance, high thoracic lesions may present with levels in the upper lumbar segments. The somatotopic distribution of fibers in the lateral spinothalamic tract, with the lowest segments represented more superficially, has been invoked to explain this apparent discrepancy. More reliable band-like radicular pain or segmental paresthesias may occur at the level of the lesion and may be of localizing value for the appropriate spinal level. If the pain is cervical, it radiates to the arms; if thoracic in origin, it is circumferential to the chest or abdomen; and if lumbar or sacral, it radiates to the legs. Localized vertebral pain (over the vertebral spinous process), which is accentuated by palpation or vertebral percussion, may occur with destructive lesions (especially infections and tumors) and may also be of localizing value. Pain that is worse when recumbent and better when sitting or standing is common with malignancy.
Defects in pain and temperature sensation below a certain level in the trunk are almost always a sign of spinal cord disease. However, because of the somatotopic organization of sensory fibers in the spinothalamic tract at higher levels, rarely a lateral medullary or lateral pontine lesion may cause a sensory deficit in the contralateral leg, trunk, or both to a specific level [83]. For example, a very laterally placed medullary lesion may damage the sacral and lumbar afferent fibers of the lateral spinothalamic tract but spare the more medial thoracic and cervical afferent fibers, resulting in a sensory loss below a specific lumbar level. A similar sensory level described with a parietal lesion is even more unusual [22].
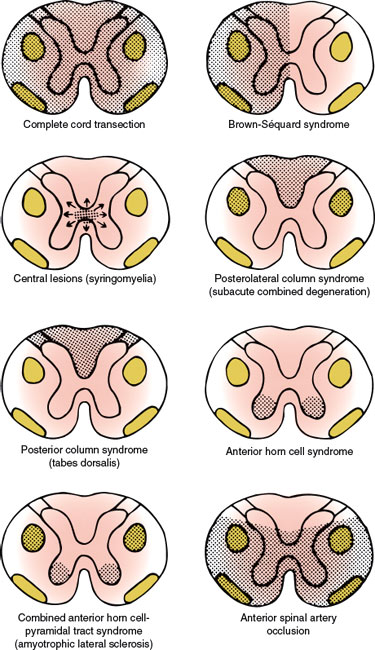
FIG. 5.3. Spinal cord syndromes.
MOTOR DISTURBANCES
Paraplegia or quadriplegia occurs below the level of the lesion due to an interruption of the descending corticospinal tracts. Initially, especially with acute lesions, the paralysis is flaccid and areflexic because of spinal shock. Eventually, hypertonic, hyperreflexic paraplegia or tetraplegia occurs with bilateral extensor toe signs, loss of superficial abdominal and cremasteric reflexes, and extensor and flexor spasms. Extension at the hip and knee occurs with incomplete or high spinal cord lesions, whereas flexion at the hip and the knee occurs with complete and lower lesions of the spinal cord.
At the level of the lesion, there are lower motor neuron signs (paresis, atrophy, fasciculations, and areflexia) in a segmental distribution because of damage to the anterior horn cells or their ventral roots. These lower motor neuron signs, which may be quite subtle in thoracic lesions, localize the lesion to a specific spinal cord level.
AUTONOMIC DISTURBANCES
Urinary and rectal sphincter dysfunction with incontinence may occur with transverse myelopathy. Urgency of micturition is the usual bladder symptom, with urinary retention a later problem, and incontinence not seen until very late. Constipation is the most common bowel symptom. Initially, atonic and, later, spastic rectal and bladder sphincter dysfunction occurs with lesions at any spinal level. Bladder and bowel dysfunction result from bilateral lesions, which may also cause orthostatic hypotension. Anhidrosis, trophic skin changes, impaired temperature control, and vasomotor instability are seen below the level of the lesion. Sexual dysfunction (especially impotence) may be present. A lesion involving the cell bodies of the preganglionic sympathetic neuron located in the cervicothoracic cord may result in an ipsilateral Horner syndrome. After the phase of spinal shock, individuals with cervical and high thoracic (above T6) acute spinal cord injuries, may develop autonomic dysreflexia.
Hemisection of the Spinal Cord (Brown-Séquard Syndrome)
Functional hemisection of the spinal cord results in a characteristic syndrome (the Brown-Séquard syndrome, Fig. 5.3) [64], which consists of the following signs and symptoms:
Loss of pain and temperature sensation contralateral to the hemisection due to interruption of the crossed spinothalamic tract. This sensory level is usually one or two segments below (caudal) the level of the lesion.
Ipsilateral loss of proprioceptive function below the level of the lesion due to interruption of the ascending fibers in the posterior columns (dorsal funiculi). Tactile sensation may be normal or minimally decreased [107].
TABLE 5.2 Brown-Séquard Syndrome

Ipsilateral spastic weakness with hyperreflexia and Babinski sign caudal to the level of the lesion due to interruption of the descending corticospinal tract.
Segmental lower motor neuron (segmental weakness and atrophy) and sensory signs (segmental anesthesia) at the level of the lesion due to damage of the anterior horn cells and dorsal rootlets at this level.
Ipsilateral loss of sweating caudal to the level of the lesion due to interruption of descending autonomic fibers in the ventral funiculus, and Ipsilateral Horner syndrome, if the lesion is cervical, and Ipsilateral hemidiaphragmatic paralysis due to damage of the upper motor neuron pathways for breathing, if the lesion is high cervical.
The Brown-Séquard syndrome is characteristically produced by extramedullary lesions (Table 5.2).
Lesions Affecting the Spinal Cord Centrally
The central spinal cord syndrome is best exemplified by syringomyelia, hydromyelia, hematomyelia, and intramedullary cord tumors (Table 5.3). The clinical course of syringomyelia is usually slowly progressive, and the syrinx rarely remains limited. Often the condition is associated with hindbrain abnormalities as seen in Chiari type I and type II or Dandy–Walker malformations, or as a late sequel to traumatic paraplegia or quadriplegia, spinal trauma, spinal cord tumors, arachnoiditis or, rarely, with myelitis [108]. Acute atypical presentations are rarely seen [4]. Spinal cord damage starts centrally and spreads centrifugally to involve other spinal cord structures. Characteristically, the decussating fibers of the spinothalamic tract conveying pain and temperature sensation are compromised initially. This results in thermoanesthesia and analgesia in a “vest-like” or “suspended” bilateral distribution with the preservation of soft touch sensation and proprioception (dissociation of sensory loss). With forward extension of the disease process, the anterior horn cells become involved at the level of the lesion, resulting in segmental neurogenic atrophy, paresis, and areflexia. Lateral extension results in an ipsilateral Horner syndrome (due to involvement of the ciliospinal center of Budge with C8–T2 lesions), kyphoscoliosis (due to involvement of the dorsomedian and ventromedian motor nuclei supplying the paraspinal muscles), and, eventually, spastic paralysis below the level of the lesion (owing to the corticospinal tract involvement). Dorsal extension disrupts dorsal column function (ipsilateral position sense and vibratory loss), and with extreme ventrolateral extension, the spinothalamic tract is affected, producing thermoanesthesia and analgesia below the spinal level of the lesion. Because of the lamination of the spinothalamic tract (dorsomedial cervical sensation and ventrolateral sacral sensation), sacral sensation is spared (sacral sparing) by intraparenchymal lesions. Syringomyelia may also occasionally cause a neuropathic arthropathy of the shoulder, elbow, and other joints [123]. Pain is present in approximately half of the patients. The fact that the initial manifestation of syringomyelia may be a neuropathic arthropathy is of major clinical importance [7].
TABLE 5.3 Central Spinal Cord Syndrome
An acute cervical central spinal cord syndrome can occur after severe hyperextension injuries of the neck [115]. Patients with this syndrome become quadriplegic after cervical trauma but regain strength in the legs in a matter of hours or even minutes. There is bladder dysfunction, usually urinary retention, and patchy and variable sensory loss below the level of the lesion. There is upper motor neuron distribution weakness more pronounced in the arms (“man-in-a-barrel syndrome”). Muscle stretch reflexes are initially absent. Considerable recovery is common. This syndrome is probably due to the damage to the central gray matter and lateral corticospinal tract at the cervical spinal cord enlargement.
Posterolateral Column Disease
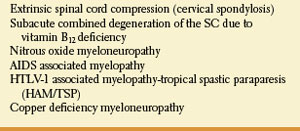
The posterior and lateral columns in the upper spinal cord may be selectively damaged in subacute combined degeneration of the spinal cord due to vitamin B12 (cobalamin) deficiency [54] and numerous other conditions (Table 5.4). Protean disorders can result in cobalamin deficiency. These include pernicious anemia due to autoimmune parietal cell dysfunction (associated with defective gastric secretion and absence of intrinsic factor), rare congenital disorders, nutritional deficiencies with inadequate dietary intake (vegans), atrophy of the gastric mucosa, partial or total gastrectomy, functionally abnormal intrinsic factor, inadequate proteolysis of dietary cobalamin, insufficient pancreatic protease, bacterial overgrowth in the intestine, terminal ileum disease, tapeworm infection, disorders of plasma transport of cobalamin, dysfunctional uptake and use of cobalamin by cells, and nitrous-oxide administration. Vitamin B12 deficiency may also be found among malnourished infants, and in offsprings of strict vegan mothers.
TABLE 5.4 Posterolateral Column Syndrome
The most common cause of cobalamin deficiency is pernicious anemia. Classic pernicious anemia produces cobalamin deficiency due to failure of the stomach to secrete intrinsic factor. Pernicious anemia is also associated with other autoimmune diseases, such as Addison’s disease, Graves’ disease, and hypoparathyroidism. In pernicious anemia, the neurological manifestations reflect myelin degeneration of the dorsal and lateral columns of the spinal cord, peripheral nerve dysfunction, and cerebral dysfunction.
Pathologic changes in cases of subacute combined degeneration predominantly involve the cervical cord, although changes may extend to the thoracic and lumbar cord regions. Microscopically, the spinal cord shows multifocal vacuolated and demyelinating lesions in the posterior and lateral columns. In longstanding cases, the posterior and lateral funiculi appear sclerotic and pale. The lesions spread laterally and longitudinally. The fibers with the largest diameters are preferentially affected. Most patients with subacute combined degeneration complain of paresthesias in the feet and, less often, in the hands, difficulties with gait and balance, and have signs of dorsal column dysfunction, including loss of proprioception and vibration sense in the legs as well as sensory ataxia with a positive Romberg’s sign and bladder atony. Pain and temperature sensations remain intact because of the preservation of the spinothalamic tracts. Loss of position sense in the second toe and loss of vibratory sense for a 256-Hertz but not a 128-Hertz tuning fork are the earliest signs of dorsolateral column involvement. Bilateral corticospinal tract dysfunction results in spasticity, hyperreflexia, and bilateral Babinski signs. However, the ankle reflexes may be lost or become hypoactive early in the process because of superimposed peripheral neuropathy. Myelopathic signs tend to be symmetric.
Nitrous oxide has multiple deleterious effects on cobalamin metabolism. Patients with unrecognized cobalamin deficiency may be particularly susceptible to brief exposures to nitrous oxide, which inactivates cobalamin-dependent methionine synthase and may cause a myeloneuropathy. In healthy subjects, this side effect on the methionine synthase methylcobalamin complex may be well compensated for by the large vitamin B12 stores in the liver and bone marrow. For patients with a preexisting vitamin B12 deficiency, even a short course of nitrous oxide anesthesia may deplete the few remaining stores. Furthermore, inactivation of methionine synthase by nitrous oxide may be more rapid in patients with low concentrations of vitamin B12 [81].
Posterior and lateral spinal cord involvement is also seen in cases of vacuolar myelopathy associated with acquired immunodeficiency syndrome (AIDS), human T-lymphotropic virus type 1 (HTLV-1), associated myelopathy (tropical spastic paraparesis), extrinsic cord compression (e.g., cervical spondylosis), copper deficiency myelopathy, and a variety of spinocerebellar ataxias.
The most common cause of spinal cord disease in patients with AIDS is AIDS-associated myelopathy. AIDS-associated vacuolar myelopathy frequently presents late in the course of HIV disease. Patients usually have a slowly progressive spastic paraparesis with brisk muscle stretch reflexes and bilateral extensor plantar responses, sensory ataxia with impaired vibration and position sense, normal cobalamin and folate levels, and increased CSF protein with pleocytosis. There may be associated dementia and a spastic bladder [129]. Gait is spastic, ataxic, or ataxo-spastic.
HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic viral immune-mediated disorder of the spinal cord caused by the human lymphotrophic virus (HTLV-1). Worldwide, millions of people are infected. Findings commonly encountered with HTLV-1-associated myelopathy include backache, leg paresthesias, urinary frequency, a slowly progressive paraparesis, muscle stretch hyperreflexia, Babinski’s signs, and impaired vibratory and position sense. CSF shows lymphocytic pleocytosis, elevated CSF protein, normal glucose content, and increased CSF IgG with antibodies to HTLV-1.
Copper absorption in humans seems to occur in the stomach and duodenum. A chronic progressive spastic-ataxic gait with proprioceptive deficits has been described in copper deficiency myelopathy (copper deficiency myeloneuropathy). Affected patients often exhibit a myelodysplastic picture with anemia, neutropenia, and thrombocytopenia, and persistently increased serum zinc levels. Serum vitamin B12, folate, and methylmalonic acid levels are normal. Serum copper and ceruloplasmin levels are low. MRI may show diffuse increased signal intensity in the dorsolateral funiculi [65–68,110]. Copper deficiency myelopathy resembles the clinical picture of subacute combined degeneration of the spinal cord secondary to cobalamin deficiency. Copper deficiency may be a delayed complication of gastric surgery [68].
Posterior Column Disease
The posterior columns are selectively damaged by tabes dorsalis (tabetic neurosyphilis, progressive locomotor ataxia). Inflammation and degeneration of the dorsal roots cause secondary destruction of the posterior columns of the spinal cord. Tabes dorsalis usually develops 10 to 20 years after onset of luetic infection and results in impaired vibration and position sense and decreased tactile localization. The lower extremities are more affected than the upper. Patients often complain of increased unsteadiness in darkness. There is sensory ataxia, noted first at night or in the dark, and a positive Romberg sign due to proprioceptive interruption; the swaying begins as soon as the eyes are closed and occurs in all directions [72].
The gait is ataxic, and “stomping” or “double tapping.” Patients often look straight ahead when walking. The gait disorder is much more pronounced in darkness or with eye closure because visual cues can no longer be incorporated in maintaining balance. Often patients fall forward immediately following eye closure (wash-basin sign or positive “sink” sign). Lightning pains are the hallmark of tabes dorsalis [48]. Patients often complain of sudden, brief, sharp, lancinating jabs most frequently in the legs, develop urinary incontinence, and have absent patellar and ankle reflexes. The affected limbs are hypotonic but not weak. Hyperextensible joints are common. Abdominal crises mimicking a surgical abdomen, occur in approximately 10 percent of patients. Laryngeal crises with stridor, rectal, and vesical crises are less common. Trophic disturbances result in neurogenic arthropathies (Charcot joints), which are analgesic joints that disintegrate and become deformed as a result of chronic trauma. Many patients have diminished pain sensation demonstrated by insensibility to pressure of the Achilles tendon (Abadie sign). Often, there is impaired light touch perception in the Hitzig zones (e.g., the central area of the face, the nipple area, the ulnar borders of the arms, the peroneal borders of the legs, and the perianal area). Many patients with tabes dorsalis also have small, miotic, and irregular pupils, unreactive to light, but normally reactive to accommodation (Argyll Robertson pupils), optic atrophy, eyelid ptosis, or ophthalmoplegia. With dysfunction of the posterior columns in the cervical region, neck flexion may elicit a sudden “electric-like” sensation down the back or into the arms (Lhermitte’s sign or “barber’s chair syndrome). Voiding dysfunction results from disturbed bladder sensation and sacral and suprasacral lesions innervating the detrusor muscle [44]. Merritt and Adams [85] described a triad of symptoms (lightning pains, ataxia, and dysuria) along with a triad of signs (Argyll-Robertson pupils, areflexia, and loss of proprioception).
Truncal and gait ataxia may occur with spinal cord lesions (e.g., metastatic tumor causing cord compression) without associated proprioceptive difficulties (e.g., the heel-to-shin test is normal). This truncal ataxia may be due to impaired conduction in the dorsal spinocerebellar tract and subsequent mismatched vermal integration of ventral spinocerebellar tract information (copy of efferent central instruction) and dorsal spinocerebellar tract information (afferent truncal feedback) [41].
Patients may present with ataxia as the primary manifestation of epidural spinal cord compression. In these patients, lower extremity dysmetria, gait ataxia, or both may be the only neurologic signs; patients usually have thoracic spine compression, suggesting possible selective vulnerability of the spinocerebellar tracts in the thoracic spine to compressive ischemia [41]. Salient disorders associated with the posterior column syndrome are summarized in Table 5.5.
TABLE 5.5 Posterior Column Syndrome










