64 Spinal Cord Tumor Resection
I. Key Points
– Intradural tumor resection has the potential for significant neurologic morbidity.1
– Prognosis and outcome are highly variable and dependent on pathology.1,2
II. Indications
– Diagnosis or treatment of a contrast-enhancing intradural lesion in a symptomatic patient
• Sensory or motor deficits and sphincter dysfunction
• Localized pain, especially nonmechanical pain exacerbated by recumbency
• Not indicated for transverse myelitis, multiple sclerosis, or drop metastases
• In malignant lesions, consider biopsy and adjuvant therapy to avoid neurologic morbidity.
III. Technique
– Place patient in prone position with Mayfield clamp (Integra LifeSciences, Plainsboro, NJ).
– Preoperative corticosteroids and broad-spectrum antibiotics are routinely administered.
– Neuromonitoring is required.
• Continuous somatosensory evoked potentials (SSEPs)
• Pre-positioning motor evoked potentials (MEPs) provide a baseline that can be used throughout the case.
– Standard midline incision with subperiosteal dissection of the paraspinal muscles.
– Laminectomy or laminoplasty is performed one level above and one level below the rostral and caudal poles of the tumor.
• Immaculate hemostasis and placement of moist Cottonoids (Saramall, Tandil, Argentina) in the epidural space will prevent the accumulation of blood in the operative cavity.
– Midline durotomy is performed just rostral to the tumor and extended caudal to the tumor (Fig. 64.1).
• Ultrasound prior to the dural opening may help define the location of the tumor.
• Use tack-up sutures to tent the dura laterally to the paraspinal muscles.
• The arachnoid is preserved and opened separately under microscopic guidance.
• The arachnoid can be clipped to the dural edges using small vascular clips.
– Locate midline to minimize neurologic morbidity.
• The tumor may distort the cord; thus, the posterior median sulcus may be estimated by inspecting the bilateral dorsal root entry zones or identifying the convergence of small vessels in the midline.
• Dorsal column mapping by monitoring SSEPs and directly stimulating the cord may be helpful.
– A midline myelotomy is started in the area of maximum cord enlargement.
• Extend the incision superiorly and inferiorly to expose the tumor in its entirety.
– Begin dissecting the tumor at the area of maximal enlargement (Fig. 64.2).
• Carefully spread the posterior columns with a micro-dissector.
• Pial sutures may be used at the edge of the incision to provide gentle traction.
– Once the tumor is exposed, send a specimen for frozen section pathology.
• High-grade tumors are debulked; postoperative adjuvant therapy is required.
• Low-grade glial tumors and ependymomas are more aggressively approached
– En bloc resection is recommended when possible.2
• Reduces intralesional bleeding and maintains a better surgical plane
– Large tumors may require piecemeal resection.
– To resect, gently push the spinal cord away from the lesion using micro-instruments.
• Minimize movement of the intact spinal cord to prevent injury.
• An ultrasonic aspirator may be useful.
– Note: dissection/resection along the median raphe may be difficult.
• Beware of coagulating the anterior spinal artery or its branches.
– Post-resection ultrasound may be valuable in determining the extent of resection.
– Irrigate inside the thecal sac to remove all blood products.
– Immaculate watertight dural closure is needed.
• Consideration can be given to dural patches/grafts to enlarge the diameter of the thecal sac in patients with expansible lesions and local swelling.
– Dural sealant should be considered.
– If a laminoplasty is called for, replace the lamina using pre-drilled mini-plates.
• This may decrease the incidence of cerebrospinal fluid (CSF) leak.
– Irrigate and close the incision in a watertight fashion.
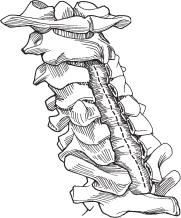
Fig. 64.1 The durotomy is completed and the arachnoid is incised. (From Vaccaro AR, Albert TJ, Spine Surgery: Tricks of the Trade 2nd ed, Thieme; Fig. 4.2.)
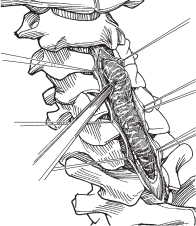
Fig. 64.2 Tumor dissection is initiated in the middle portion of the tumor, which is the bulkiest. (From Vaccaro AR, Albert TJ, Spine Surgery: Tricks of the Trade 2nd ed, Thieme; Fig. 4.3.)
IV. Complications
– Neurologic injury
• Significant motor morbidity
• Significant proprioceptive morbidity
• Sphincter dysfunction (can be minimized if the conus is avoided)
– CSF leak
– Wound infection
• Increases with adjuvant cytotoxic agents
– Post-laminectomy kyphosis
• Greatest risk: children <3 years, patients with preoperative deformity, patients with preoperative neurologic deficit
• Laminoplasty may reduce this risk.
V. Postoperative Care
– Place patient supine and require bed rest for 24 to 48 hours as a CSF leak precaution.
– Patient can be weaned from steroids over 2 to 4 weeks.
– Consider postoperative magnetic resonance imaging (MRI) with and without contrast within 48 hours of surgery.
• Consider reoperation for residual tumor (depending on pathology and neurologic status).
– Gradually mobilize the patient with the assistance of physical therapy.
• Most patients will have sensory changes that will make ambulation difficult.
– Leave Foley catheter in place until patient is ambulatory.
• Straight catheterization is needed for sphincter dysfunction.
– Patient can be discharged to home or rehab facility depending on functional status once discharge criteria are met (typically, tolerating diet, having adequate pain control on oral medications, and, depending on functional status, voiding and ambulating).
– Depending on the pathologic findings, the patient should have a consultation with radiation oncology and neurologic oncology personnel to discuss adjuvant therapy.
• No radiation or chemotherapy should be given until the wound is healed, which typically requires 3 to 4 weeks.
VI. Outcomes
– Highly dependent on tumor pathology and the availability of adjuvant therapies
– A large proportion of patients require inpatient rehabilitation.
– In the event of recurrence, consider further surgery.
VII. Surgical Pearls
– Detailed preoperative discussion of risks is mandatory.
• Postoperative neurologic deficit is common and unpredictable.
• Dorsal column dysfunction is expected.
– Neurologic monitoring is mandatory.
• Epidural MEPs can help prevent neurologic injury.
– Laminoplasty may decrease the risk of postoperative CSF leak and kyphosis.
• Place mini-plates on lamina and drill pilot holes before performing the laminectomy to ease repair.
– Begin dissection at the midpoint of the lesion, where it is the bulkiest, to reduce injury.
– In lesions with associated cysts, the laminectomy need not extend beyond the solid tumor.
• Cyst walls are typically nonneoplastic, and complete tumor resection results in cyst resolution.
Common Clinical Questions
1. Where and in what direction should the myelotomy be performed?
2. If the tumor has a cystic component, does this need to be resected?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







