Chapter 10 Spinal Fluid Examination
History
In 1891, Wynter first reported the use of LP as a therapeutic measure after using the technique in a patient with tuberculous meningitis. The diagnostic value of LP was first recognized and described by Quincke in 1891. His innovations included using a percutaneous needle with a stylet, a manometer to record the opening pressure, and measurements of CSF cell counts, protein concentration, and sugar content. The technique quickly gained acceptance and had become a routine part of medical care by the turn of the 20th century. Several authors have summarized this early history [Vandam, 1989]. Numerous subsequent investigators have contributed to an ever-expanding understanding of the value of CSF analysis in various neurologic diseases [Fishman, 1992].
Cerebrospinal Fluid Formation, Flow, and Absorption
CSF is principally formed by secretion from the choroid plexus, villous invaginations of the walls of the lateral, third, and fourth ventricles which are richly vascularized and lined by a ciliated epithelium (Figure 10-1). The choroid plexus of the lateral ventricles is continuous through the foramina of Monro with the choroid plexus of the roof of the third ventricle. The arterial supply to this portion of the choroid plexus originates from the anterior choroidal arteries, which branch off the internal carotid artery, and from the posterior choroidal arteries, which are branches of the posterior cerebral arteries. The choroid plexus within the fourth ventricle, which sends extensions through the lateral foramina of Luschka, is usually supplied by the posterior inferior cerebellar arteries. In studies of rats, blood flow to choroidal vessels is almost 10 times greater than that to the cerebral cortex [Szmydynger-Chodobska et al., 1994]. Nerves derived from several sources, including the cervical sympathetic chain, the neural plexus of the internal carotid and posterior cerebral arteries, and the vagal nuclei, provide extensive adrenergic, cholinergic, and peptidergic innervation of the blood vessels and epithelial cells of the choroid plexus. These inputs have an influence on the rate of CSF production that is independent of vasomotor changes [Lindvall and Owman, 1981].
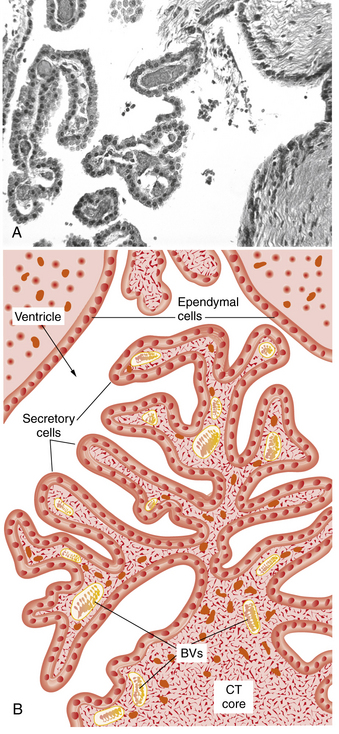
Fig. 10-1 Structure of the choroid plexus.
(A, Courtesy of Dr. Thomas Caceci, Virginia–Maryland College of Veterinary Medicine. B, Adapted from a drawing by Dr. Samir El-Shafey, Virginia–Maryland College of Veterinary Medicine.)
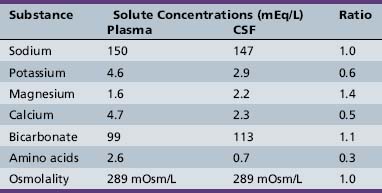
The capillaries of the choroid plexus, unlike those found in most other areas of the brain, have large fenestrations that offer little resistance to the passage of fluid, ions, and small macromolecules [Segal, 1993]. Passage of blood past these capillaries creates an ultrafiltrate of plasma within the interstitial space at the basolateral surface of the epithelial cells. Analysis of the ionic composition of CSF relative to plasma (Table 10-1) reveals that this interstitial fluid is modified considerably before it reaches the ventricular system, and strongly supports CSF formation being an active secretory process [Davson et al., 1987].
Additional studies have found that CSF secretion depends highly on active transport proteins that, as with other secretory epithelia, are differentially localized on the apical and basolateral membranes of the choroid plexus epithelial cells. Water follows the flow of sodium and chloride ions from the interstitial fluid into the CSF. Carbonic anhydrase within the epithelial cells catalyzes the formation of carbonic acid from water and carbon dioxide. Carbon dioxide diffuses freely, but the dissociation products of carbonic acid, bicarbonate and hydrogen, are transported across the basolateral membrane by specific proteins in exchange for sodium and chloride, favoring the passive diffusion of water into the cells [Speake et al., 2001]. Sodium transport out of the cells is principally mediated by sodium–potassium ATPases on the apical membrane, with selectively permeable apical chloride and potassium channels rounding out the complement of transporters thought to be most important to creating a net flow of water into the CSF (Figure 10-2). Water does flow passively along osmotic gradients through the choroid plexus, moving freely through the aquaporin-1 channels which are highly expressed on the apical surface of the epithelial cells [Tait et al., 2008]. However, the contribution to CSF production from osmosis is minimal, as CSF production by experimental animals is unaltered even by instillation of high osmolar fluid into the ventricles [Macaulay and Zeuthen, 2009].
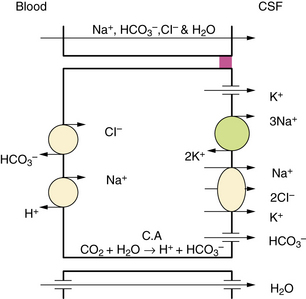
Fig. 10-2 Major processes involved in cerebrospinal fluid (CSF) secretion.
(From Brown PD, Davies SL, Speake T, et al. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 2004;129:957.)
Other specific active transport proteins present along the basolateral membrane allow transport into the CSF of essential hydrophilic micronutrients, including glucose, amino acids, purines, nucleosides, and vitamins. Transport proteins along the apical membrane work to clear the CSF of potentially toxic metabolites, such as organic acids and bases [Spector and Johanson, 1989].
Tight junctions link the choroid plexus epithelial cells, limiting the free diffusion of ionic molecules and creating the blood–CSF barrier. Although protein diffusion is largely restricted, most of the small amount of protein found in the CSF is nevertheless of plasma origin. Maintenance of a relatively stable CSF composition, despite wide variation in the composition of the plasma, reflects the integrity of the blood–CSF barrier and the work of active transporters against concentration gradients [Fishman, 1992].
The choroid plexus produces from 70 to 90 percent of the CSF, with the remainder deriving from movement of brain parenchymal interstitial fluid across the ependyma into the ventricles and across the meninges into the subarachnoid space [Proescholdt, 2000]. The rate of CSF formation in healthy adults averages 0.35 mL per minute, or roughly 500 mL each day. Children produce proportionally less CSF, depending on their height and weight, with as little as 25 mL produced per day in newborns. Postmortem studies have provided estimates that total CSF volume ranges from 50 mL in term neonates to 150 mL in adults, with only a small percentage contained within normal-sized ventricles. The total volume of the CSF undergoes complete replacement 3–4 times each day.
Choroid plexus epithelial cells express a wide range of receptors for hormones and neurotransmitters [Nilsson et al., 1992], but the rate of CSF formation appears to remain fairly constant under most conditions. Many factors have been found to influence the rate of CSF production in animal models, including cholera toxin, norepinephrine, hyperosmolality, hypothermia, atrial natriuretic hormone, vasopressin, serotonin, and dopamine [Rosenberg, 1990]. Cholera toxin and norepinephrine increase CSF production through an increase in cyclic AMP formation, which has been linked to increased bicarbonate transport [Saito and Wright, 1984].
Greatly reduced CSF production has been seen after experimental administration of the sodium–potassium ATPase inhibitor ouabain [Vates et al., 1964]. Carbonic anhydrase inhibitors, such as acetazolamide, can reduce the choroidal production of CSF in rats by 50–100 percent [Fishman, 1992]. Of the seven mammalian isoforms of carbonic anhydrase, however, the CAIII isoform that is most abundant in the human choroid plexus is insensitive to acetazolamide [Nogradi et al., 1993], explaining the somewhat limited clinical effectiveness of this medication [Cowan and Whitelaw, 1991]. Furosemide and other loop diuretics weakly inhibit carbonic anhydrase activity but are thought to reduce CSF production through inhibition of sodium, potassium, and chloride co-transporters [Johanson et al., 1994]. Endogenous vasopressin release and V1 receptor activation appears to mediate some of the reduction in choroidal blood flow and CSF formation that occurs with hypoxia or with increased intracranial pressure [Faraci et al., 1994]. Elevated CSF vasopressin levels have been found in some patients associated with the syndrome of idiopathic intracranial hypertension [Sorensen et al., 1982].
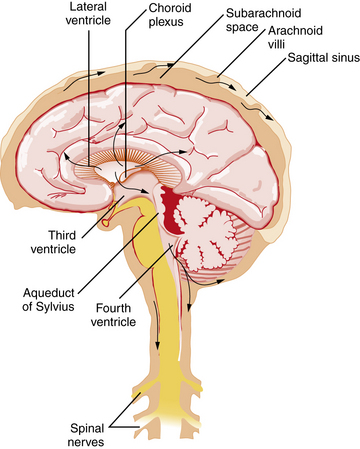
Figure 10-3 illustrates the pathway taken by the CSF. Fluid is secreted into the lateral ventricles, passes through the foramina of Monro into the third ventricle, and flows through the aqueduct of Sylvius into the fourth ventricle. CSF exits the ventricular system through the lateral foramina of Luschka to enter the prepontine cistern and cerebellopontine angles. Alternatively, CSF can leave the fourth ventricle through the midline foramen of Magendie, entering the cisterna magna, from which it can flow upward over the cerebellar hemispheres or downward into the spinal subarachnoid space and around the brainstem into the basal cisterns, including the interpeduncular cistern. CSF flows predominantly downward posterior to the spinal cord and upward anterior to the cord, leading the fluid eventually to reach the basal cisterns, from which flow is mainly upward over the brain convexity. The quality of CSF flow can be visualized noninvasively using spin-echo labeled magnetic resonance imaging (MRI) [Yamada et al., 2008].
In the steady state, the rate of CSF absorption is equal to that of its production. Most CSF is absorbed into the venous sinuses across the arachnoid villi and granulations, arachnoid membrane invaginations through the dural lining of the sinuses that are concentrated near the sagittal sinus. Absorption across the arachnoid villi occurs by vesicular transport. This action demonstrates a dependence on the hydrostatic pressure gradient across the villous surface, such that the villi act as one-way pressure valves that open above a threshold pressure, usually 20–50 mm H2O [Welch and Friedman, 1960]. Some CSF absorption occurs across the capillaries, veins, and lymphatics of the spinal cord and spinal roots [Koh et al., 2005], and evidence suggests that CSF is also reabsorbed through the cribriform plate and nasal lymphatics [Johnston et al., 2004].
Cerebrospinal Fluid Function
The CSF provides buoyancy and physical protection to the brain, absorbing the stretching and compressive forces generated by normal head movement and lessening the impact of the deceleration and rotational forces created by head trauma. A second protective function derives from the ability of the CSF to redistribute in response to acute changes in other intracranial contents, helping to maintain normal intracranial pressures. The CSF is also thought to serve as a route for the transport of centrally acting hormones, such as for the diffusion of hypothalamic releasing factors across the third ventricle [Kozlowski, 1982].
The CSF aids in the excretion of metabolites through bulk absorption of the interstitial fluid of the brain and spinal cord, with which it is in close communication [Go, 1997], prompting speculation that the CSF pathways are analogous in function to the lymphatic channels that these tissues lack [Cserr et al., 1977]. This “sink action,” along with the various mechanisms by which the CSF composition is maintained, suggests that the CSF plays a significant role in normal physiology and in the compensatory response to pathologic situations [Hochwald, 1984].
Diagnostic Sampling of Cerebrospinal Fluid
Contraindications and Cautions
LP through a soft tissue infection such as cellulitis is discouraged out of concern that this can introduce bacteria into deeper tissues and cause an epidural abscess, osteomyelitis, or meningitis [Abolnik et al., 1995]. An increased bleeding tendency is also a contraindication because puncture may incur a greater risk of epidural, subdural, or subarachnoid bleeding [Adler et al., 2001]. However, coagulopathy may be correctable by stopping an anticoagulant medication or by giving an appropriate transfusion of platelets or clotting factors [Silverman et al., 1993]. Before LP is performed, it is believed that the patient should have a platelet count greater than 10,000/mm3 and an international normalized ratio less than 1.5 [Howard et al., 2000]. Anticoagulation therapy should probably be delayed for at least 2 hours after LP [Sadjadpour, 1977].
There is considerable and understandable fear that LP in patients with a CSF obstruction can unbalance the pressure across the area of obstruction and contribute to cerebral herniation or spinal cord compression. Neuroimaging, usually with cranial computed tomography (CT), is often performed before LP, especially in patients who appear ill. Patients are considered to be at an increased risk for cerebral herniation if neuroimaging reveals a focal mass lesion or obliteration of the fourth ventricle or quadrigeminal cistern. In adults with suspected meningitis, patients without clinical signs of increased intracranial pressure (i.e., papilledema or altered level of consciousness) or focal neurologic deficits are very unlikely to have an abnormal CT scan or to herniate after LP [Hasbun et al., 2001]. Children undergoing evaluation for suspected meningitis who are at higher risk of having a cerebral abscess, such as those with a history of cyanotic congenital heart disease, may need neuroimaging prior to LP [Tunkel et al., 2004]. However, herniation has been reported after normal neuroimaging, leading many to continue to debate the appropriate use of neuroimaging before LP [Oliver et al., 2003]. Since it is considered essential that antibiotic administration not be delayed when meningitis is suspected, especially with meningococcal disease, current recommendations are to start antibiotics as soon as possible, before LP if necessary [Tunkel et al., 2004].
Procedure
The lower end of the spinal cord, the conus medullaris, is found by MRI to be at the L1–2 vertebral interspace in most subjects and safely above the L3 vertebral body in all subjects without spinal deformity [Saifuddin et al., 1998]. To avoid damaging the conus, only the interspaces below L3 are used for LP. With a patient lying in the lateral recumbent position, the L3–4 interspace can be found at the level of the superior iliac crests. This area should be carefully palpated and marked to ensure that it can be identified after the patient is cleaned and draped. Having the patient curl with the neck and hips flexed can maximize the size of the interspace. Whether the patient is placed in a lateral recumbent or seated position, care should be taken to maintain the alignment of the shoulders and hips, avoiding rotation of the spine. Having the patient’s back near the edge of the bed and at a proper height can aid in the comfort and success of the physician. The recommended positioning is shown in Figure 10-4.
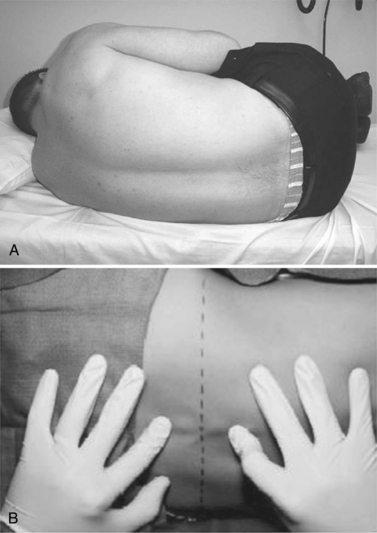
Fig. 10-4 Proper positioning of the patient.
(From Roos KL. Lumbar puncture. Semin Neurol 2003;23:105.)
The patient’s skin should be prepared using a microbicidal agent, such as a 10 percent solution of povidone-iodine. The skin should be given time to dry and then wiped clean and draped with sterile towels. A local anesthetic, such as a 1 percent solution of lidocaine, can be injected into the area to minimize the pain of repeated needlesticks. Anesthesia provides little benefit when the LP can be accomplished with a single needlestick, because the pain of the anesthetic injection often equals or exceeds that of the spinal needle insertion. A reasonable alternative is the use of a topical analgesic cream, such as the 5 percent emulsion of lidocaine and prilocaine commonly referred to as eutectic mixture of local anesthetics (EMLA). EMLA requires 20–30 minutes of application but has been associated with a decreased pain response, even in neonates [Kaur et al., 2003].
The most commonly used spinal needles are 2.5- or 5-cm long, 20- or 22-gauge Quincke needles, which are beveled and fitted with a stylet. The sharply beveled tip of the Quincke needle should be turned to face the patient’s side so that its cutting edge can push between, rather than cut, the longitudinal fibers of the dura. Atraumatic needles, such as the Whitacre and Sprotte needles, have duller tips that make dural injury less likely (Figure 10-5). These needles are also more flexible, encounter more tissue resistance, and require the use of an introducer, making them more difficult to position. Advancing the needle without its stylet in place may increase the chances of successful and nontraumatic CSF collection [Nigrovic et al., 2007], but has been discouraged because of concern that this will introduce cells that might later form a spinal epidermoid tumor [Ziv et al., 2004].
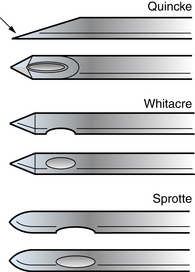
Fig. 10-5 The Quincke, Whitacre, and Sprotte needles.
The arrow indicates the sharp edge of the beveled tip of the Quincke needle.
(From Roos KL. Lumbar puncture. Semin Neurol 2003;23:105.)
The needle is gradually advanced, the ligamentum flavum and dura are pierced, and a slight popping sensation is felt as the subarachnoid space is entered. The stylet is removed to allow CSF to drain to the needle hub. If no fluid appears, the needle should be rotated a quarter-turn in case a nerve rootlet or strand of arachnoid is obstructing the opening. If this does not initiate CSF flow, the needle can be introduced a few millimeters more, sometimes resulting in a second popping sensation. If there is still no CSF, the stylet should be replaced and the needle slowly withdrawn for redirection of the needle toward the same interspace or for selection of another interspace [Roos, 2003].
Normal opening pressures in adults and older children generally range from 120 to 200 mm H2O. Pressures as high as 250 mm H2O in obese adults are also considered normal [Corbett and Mehta, 1983]. In a young, relaxed child who is in the lateral recumbent position, normal CSF pressures range from 50 to 200 mm H2O when the neck and legs are extended, but can rise to 100–280 mm H2O when the neck and legs are flexed [Clark et al., 2000]. Normal pressures in newborns vary between 90 and 120 mm H2O [Volpe, 2001]. Pathologic elevations of the CSF pressure are discussed in Chapter 77.
Replacing the stylet before withdrawing the spinal needle is recommended to avoid pulling a strand of arachnoid through the dura, creating a channel for continued CSF leakage and increasing the risk of postprocedural headache [Strupp et al., 1998]. The clinician should carefully document the details of the LP in the medical record.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree