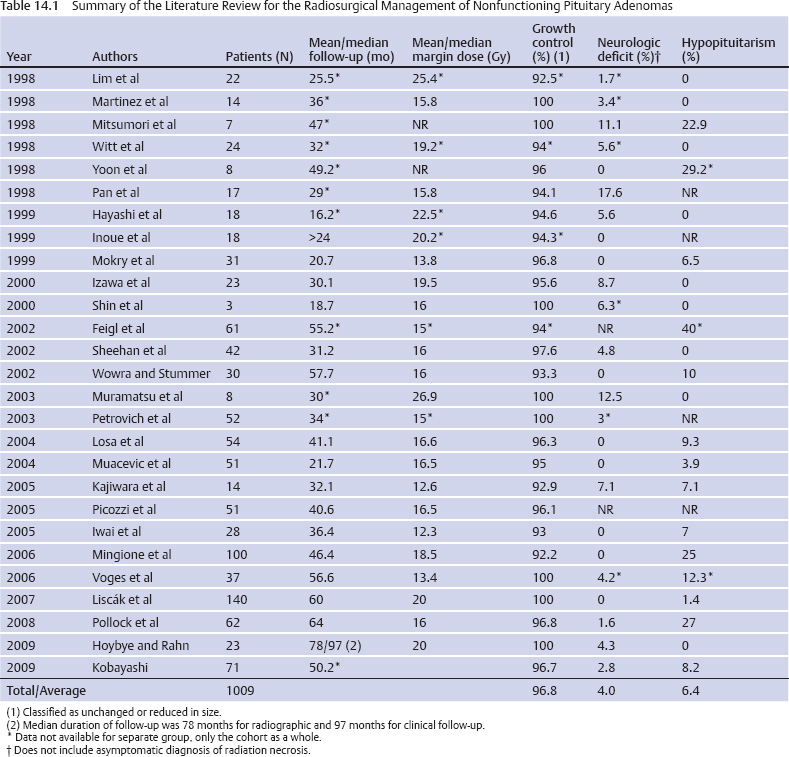
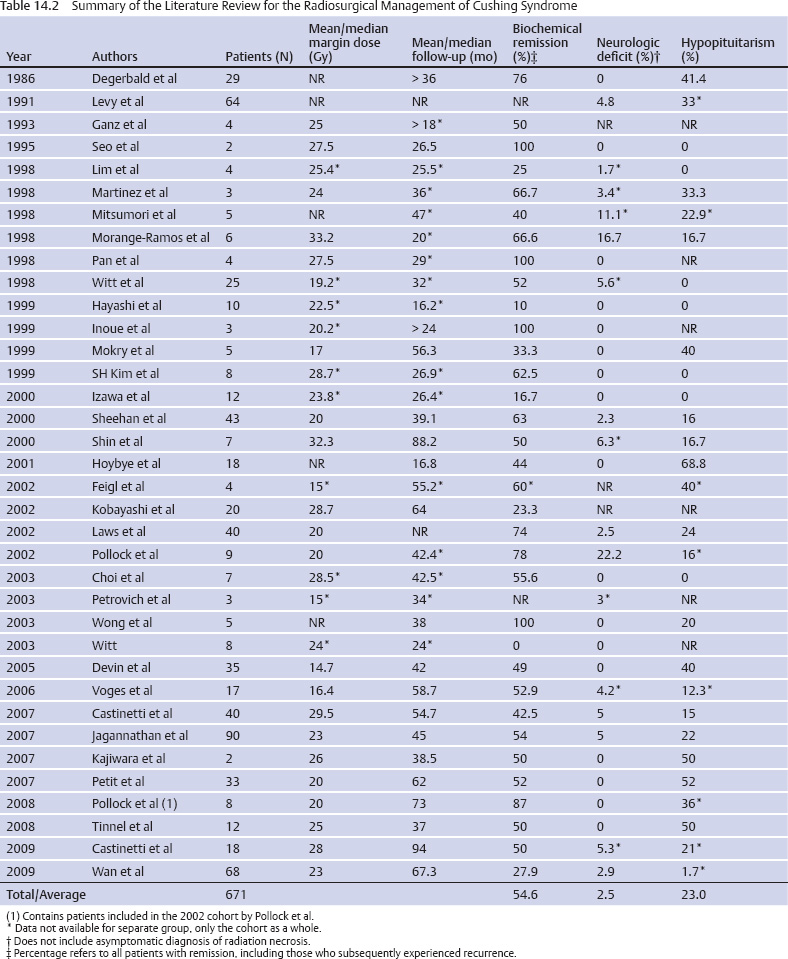
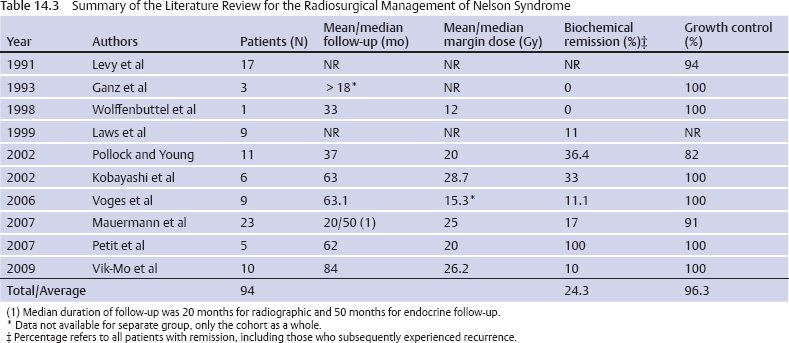
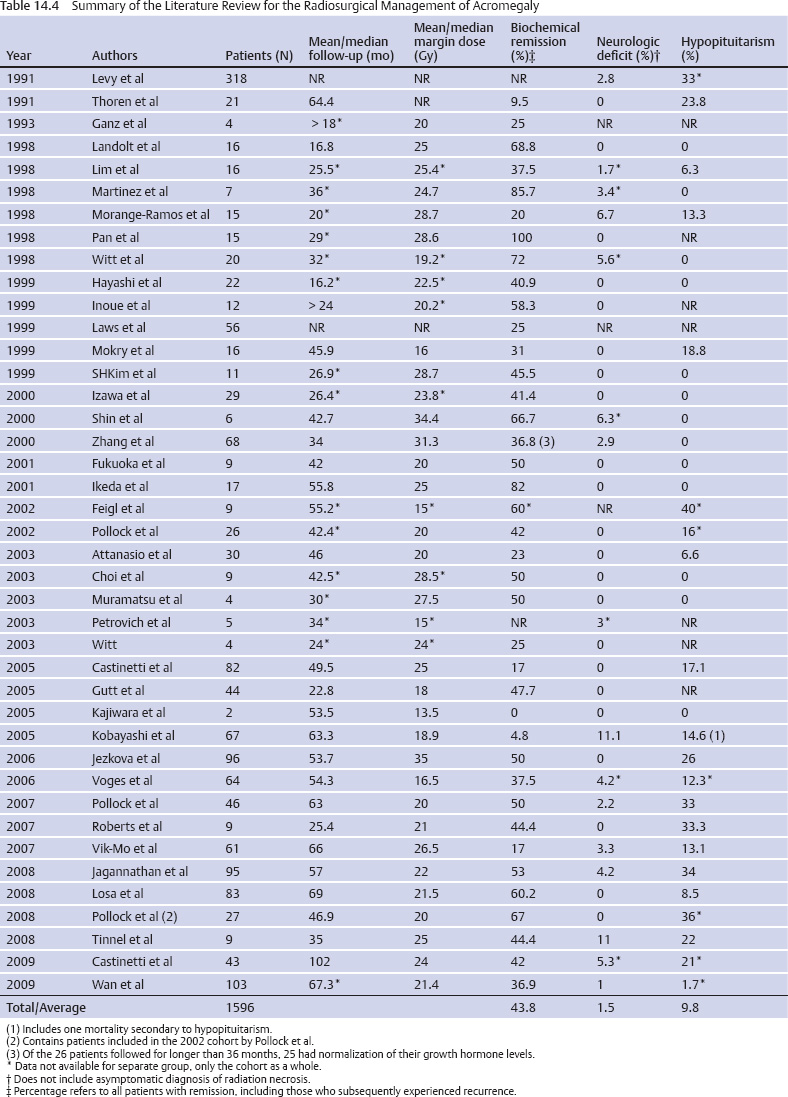
14 Stereotactic Radiosurgery Tumors in and around the sella turcica represent some of the most challenging clinical entities that clinicians have dealt with over the past century. Nearly 100 years after Harvey Cushing’s landmark work, The Pituitary Body and Its Disorders: Clinical States Produced by Disorders of the Hypophysis Cerebri,1 pituitary adenomas, meningiomas, and other, rarer tumors of the parasellar and sellar region remain difficult to cure with microsurgical techniques alone. In fact, Harvey Cushing recognized the difficulties of conventional surgical approaches for treating intracranial tumors. Cushing and colleagues used a device called a radium bomb to deliver single-session, focused radiation to intracranial tumors.2,3 At the time, the understanding of radiobiology and dosimetry was rather simplistic. Nevertheless, Cushing recognized the need for neurosurgeons to use radiation as an adjunct to the scalpel for treating intracranial tumors. In 1951, stereotactic radiosurgery (SRS) was described by Lars Leksell as the “closed skull destruction of an intracranial target using ionizing radiation.”4 Leksell treated the first patient with a pituitary adenoma to undergo surgery with the gamma knife in 1968. Since that time, SRS has been used to treat thousands of patients with sellar and parasellar tumors. Radiosurgery can be used as an upfront treatment or to achieve control of recurrent or residual tumor. Great attention and effort in the field of SRS have been placed on the preservation of surrounding neuronal, vascular, and hormonal structures. Refinement of the radiosurgical technique for parasellar and sellar tumors has been achieved through advances in radiobiology, neuroimaging, medical physics, and engineering. In this chapter, we review the role of SRS for the two most commonly treated tumors types in the parasellar and sellar region: pituitary adenomas and meningiomas. SRS involves focusing a high dose of radiation to the tumor while sparing surrounding structures significant doses of radiation. Radiosurgery is usually delivered in a single session but may be delivered in up to five sessions.5 It uses a steep falloff of the radiation dose to the surrounding tissues. Patients are immobilized with rigid frames fixed to the skull or other immobilization devices (eg, aquaplast masks or bite blocks). Radiosurgery is imageguided and generally achieves submillimeter accuracy. Integrated imaging may be used to further track and compensate for potential sources of error. There are several types of radiosurgical delivery devices, including the gamma knife, modified linear accelerators (LINACs), and proton beam units. Single-session radiosurgical doses for nonfunctioning adenomas are typically 12 to 18 Gy, and 15 to 30 Gy for functioning adenomas. Radiosurgical doses for World Health Organization (WHO) grade 1 meningiomas are typically 12 to 18 Gy. For multisession radiosurgery, these doses may be divided into two to five sessions or fractions. Gamma knife radiosurgery involves the use of multiple isocenters of varying beam diameters to achieve a dose plan that conforms to irregular three-dimensional volumes. The number of isocenters varies based upon the size, shape, and location of the adenoma. In the current version of the gamma knife, each isocenter is comprised of eight independent sectors. Beam diameters for the current Gamma Knife Perfexion unit vary from zero (ie, blocked) to 16 mm in diameter. Linear accelerator (LINAC)–based radiosurgery (eg, CyberKnife, Novalis Tx, Trilogy, Axesse) uses multiple radiation arcs to crossfire photon beams at a target.6 Most systems use nondynamic techniques in which the arc is moved around its radius to deliver radiation that enters from many different vantage points. Technical improvements with LINAC-based radiosurgery include beam shaping, intensity modulation, minileaf collimation, and onboard computed tomography or fluoroscopic imaging. Proton therapy is a method of external beam radiotherapy; it has been adapted as a radiosurgical tool for intracranial pathology. It takes advantage of the inherent superior dose distribution of protons compared with photons that is a consequence of the Bragg peak phenomenon.7 Currently, there are a few centers where this treatment is available in the United States and abroad. The number of such centers is likely to increase in the coming years as compact proton units are developed and proton beam technology is applied to more types of intracranial and extracranial disease. Pituitary adenomas are the most common intrasellar neoplasms and are found in 10 to 27% of the general population.8,9 Microadenomas (<1 cm) may be discovered incidentally during magnetic resonance imaging (MRI) or may be diagnosed when a patient has symptoms of hormone hypersecretion. Macroadenomas may be diagnosed as a result of mass effect inducing hypopituitarism, elevation in prolactin, or a neurologic deficit (eg, cranial nerve dysfunction). The distribution of functioning and nonfunctioning microadenomas is equal. Among macroadenomas, nonfunctioning lesions occur with greater frequency (~80%).8 Patients with pituitary adenomas often present with headache (40–60%), visual disturbance, hypopituitarism, or, less frequently, apoplexy.8,9 Surgical intervention is currently the mainstay of treatment for nearly all lesions except prolactinomas. Patients with large or invasive lesions often require additional therapeutic modalities, and radiosurgery is an important part of their treatment.9 Growth control following microsurgical resection will be achieved in 50 to 80% of patients with macroadenomas.10 Radiosurgery provides an excellent therapeutic option for those with continued growth or evidence of recurrence. Worldwide, more than 11,000 patients have been treated, with the published literature supporting effective control of tumor growth and low rates of complications.10 Twenty-seven series were identified comprising 1009 patients with nonfunctioning adenomas10–35 (Table 14.1). Follow-up (mean/median) ranged from 16.2 to 64 months, with an average rate of growth control of 96.8% (range, 92.2–100%; Fig. 14.1). The dose given to the tumor margin ranged from 12.3 to 26.9 gray (Gy). Neurologic deficits were uncommon (average, 4.0%; range, 0–17.6%), as was hypopitutarism (average, 6.4%; range, 0–40%). Pollock et al,29 in their series of 62 patients, reported a rate of 27% experiencing new anterior pituitary deficiencies. There was no substantial difference in the dosimetry between those with and without hypopituitarism after radiosurgery; a median margin dose of 16 Gy was used in that series. However, they did demonstrate a significant relationship with the volume of the lesion treated; patients with a tumor volume of 4.0 cm3 or less had a 5-year risk for developing a new hormonal deficit of 18%, whereas those with lesions larger than 4.0 cm3 had a 5-year risk of 58% (P = 0.02). At our institution, we reported 90% tumor control in a series of 100 patients with nonfunctioning pituitary adenomas.22 Tumor control was reduced when the margin dose to the tumor was less than 12 Gy. These data provide a basis upon which to provide patients with advice regarding the risks of intervention. In 80% of cases, endogenous Cushing syndrome results from hypersecretion of adrenocorticotropic hormone (ACTH), usually secondary to a pituitary corticotrope adenoma.36 Although surgical resection remains the primary treatment for ACTH-secreting pituitary adenomas, invasion of the surrounding dura and/or cavernous sinus or the presence of a lesion undetectable on MRI makes surgical cure improbable. In these situations, radiosurgery serves as an invaluable adjunctive modality. What defines “cure” in Cushing syndrome remains the subject of some controversy. In 2003, the Endocrine Society published a consensus statement regarding the diagnosis and treatment of this disease, including the criteria for establishing biochemical remission or cure.37 The guidelines were updated in 2008, and both versions suggest the use of morning postoperative serum cortisol levels.36 Very low levels, less than 2 mcg/dL, predict a low rate of recurrence (~10% in 10 years). Most series use either mean serum free cortisol (5.4–10.8 mcg/dL), midnight salivary cortisol (normal, –4.2 nmol/L), or a urinary free cortisol (UFC) within the normal range. Nevertheless, there is significant variability in the exact testing and levels used to define biochemical remission. A review of the radiosurgical literature revealed 36 studies comprising 671 patients with Cushing disease treated with radiosurgery, in most cases as an adjunct to operative resection11–16,18,21,23,24,27,31–33,38–59 (Table 14.2). Most used 24-hour UFC or serum cortisol to assess remission. However, the criteria for defining remission were not reported for all series. The average rate of endocrine remission was 54.6%, with a range from 0 to 100% over a follow-up period (mean/median) from 16.2 to 94 months. In our experience, endocrine remission of Cushing disease was achieved on average ~12 months after SRS.45 The rates of new neurologic deficit, including visual deterioration, were low (average, 2.5%), with some form of new anterior pituitary deficiency occurring in 23% of patients (range, 0–68.8%). Emphasizing the importance of long-term follow-up, late recurrence was also demonstrated in several series, with rates as high as 20%.45,56 In some patients, Cushing syndrome remains refractory to surgical resection and radiation therapy of pituitary lesion. In these patients, bilateral adrenalectomy remains a therapeutic option to induce remission of hypercortisolemia. Although this procedure is effective, Nelson syndrome is a well-described complication, occurring in up to 23% of patients.60 Characterized by elevated ACTH levels, hyperpigmentation, and progressive and often invasive tumors, this syndrome presents a therapeutic challenge of its own. As opposed to the literature regarding the radiosurgical treatment of other pituitary adenomas, that for the use of radiosurgery in Nelson syndrome is fairly sparse. Ten series encompassing 94 patients have been published 32,43,47,49,61–66 (Table 14.3). Over an average follow-up period (mean/median) ranging from 18 to 84 months, normalization of ACTH occurred in only 24.3%; however, a significant reduction in hormone levels was more frequent. Control of tumor growth was achieved in the majority of cases (range, 82–100%). Overall, the paucity of literature prevents strong conclusions from being drawn, but it appears as if good tumor control with a reduction in ACTH levels is achievable with radiosurgery for patients with Nelson syndrome. Acromegaly occurs with a prevalence of ~60 per million and has significant associated morbidity and mortality (hypertension, diabetes, cardiomyopathy, and sleep apnea), with a standardized mortality ratio of 1.48.67 Surgical resection is widely considered to be the first-line therapy; however, like patients with Cushing disease, those with invasion of surrounding structures (eg, the dura or the cavernous sinus) are unlikely to be cured. Although there exists some variation in the definition of biochemical cure, most consider a growth hormone (GH) level below 2.0 ng/ mL to be appropriate because it has been associated with a reversal of the morbidity and mortality of the disease.68 This GH level should occur with an insulin-like growth factor (IGF-1) level that is within the normal range for age and gender. Forty-one series have been published comprising 1596 patients with acromegaly over a follow-up period (mean/median) ranging from 16.2 to 102 months 11–16,18,21,24,26,27,31–33,40,43,46,49,50,52,53,56–58,66,69–81 (Table 14.4). The rates of endocrine remission ranged from 0 to 100% (average, 43.8%). Several factors contributed to this considerable variation, including variation in the definition of remission, use of somatostatin analogues both during treatment and follow-up, and differences in treatment parameters. Radiosurgery was associated with a low incidence of adverse effects, with a reported 1.5% of patients developing new neurologic deficits, primarily changes in visual acuity or extraocular motility. Additionally, an average of 9.8% of patients developed some form of pituitary dysfunction following SRS. Of note, radiosurgically induced endocrine remission appears to occur later than in Cushing disease. Our center observed a mean time to endocrine remission of ~24 months after radiosurgery, compared with 12 months for a similarly treated cohort of patients with Cushing disease.82 This and similar findings of a differential response of secretory pituitary adenomas to radiosurgery warrant further investigation in terms of the underlying radiobiology and ways to augment the effects of radiosurgery on more resistant types of secretory pituitary adenomas.52 Prolactinomas are the most common secretory pituitary adenomas and account for 44% of all pituitary microadenomas in autopsy series.83 Unlike with other adenomas, observation is within the management paradigm, and when treatment is indicated, medical therapy remains the first line choice for most patients with prolactinomas. Concerning the definition of biochemical cure, most consider it to be a gender-appropriate normalization of serum prolactin levels. However, some studies have demonstrated that treatment may disrupt the pituitary stalk, leading to elevation in prolactin levels and falsely lowered rates of cure.44 A review of the literature revealed 32 radiosurgical series including 765 patients with a prolactin-secreting adenoma 11–18,21,23,24,27,32,33,35,40,43,46,49,50,52,53,56,57,66,70,84–89 (Table 14.5). Biochemical cure was realized in 30.8% of patients overall (0–83.3%) over a follow-up period (mean/median) of 19.5 to 75.5 months. Growth control rates were substantially better, with many achieving rates of 90% or greater. Here, as with other secretory adenomas, the use of medical therapy both during treatment and through follow-up had an effect on outcomes. The rates of adverse events remained low, with a mean of 2.1% (range, 0–9.1%) developing new neurologic deficits and 7.5% having some form of new pituitary dysfunction (range, 0–28%). Given the morbidity associated with hypersecretory lesions, radiosurgical intervention would ideally yield hormone normalization within a period of time similar to that seen after surgical extirpation.90 Unfortunately, the period during which remission can occur is longer and demonstrates considerable variation, with reports of occurrence ranging from 3 months to 8 years.31,55,79 Concerning factors influencing remission, several groups have performed investigations designed to determine what if any variables may be used to predict or alter the efficacy of radiosurgery. Pollock et al79 evaluated 46 patients with GH-secreting adenomas and identified two significant associations in both univariate and multivariate analysis. A preoperative IGF-1 level greater than 2.25 times the upper limit of normal was significantly associated with lower rates of biochemical cure (hazard ratio [HR], 2.9; 95% confidence interval [CI], 1.2–6.9), with this finding supporting the earlier reports by Castinetti and colleagues,71 who identified a significant association between preoperative GH and IGF-1 levels and rates of remission. The perioperative use of suppressive medications was also found to have an influence on remission (HR, 4.2; 95% CI, 1.4–13.2).79 In patients with IGF-1 levels less than 2.25 times the upper limit of normal and those who were off somatostatin agonists at the time of radiosurgery, the rates of biochemical remission exceeded 80%. A similar finding was demonstrated by Landolt et al,91 who showed that the remission rates fell from 60% to 11% for those using octreotide in the perioperative setting. Mechanisms underlying this result include a decreased radiosensitivity of tumor cells secondary to decreased cell division. Additionally, octreotide may act as a free radical scavenger, thereby decreasing the damage incurred by DNA following exposure to ionizing radiation. Importantly, this result is not exclusive to acromegaly. Landolt and colleagues87 found a nonsignificant trend toward worse outcomes in patients treated with radiosurgery for prolactinomas while on dopamine agonists. Pouratian89 et al, in their analysis of 23 patients with refractory prolactinomas, demonstrated a significant increase in the rates of remission for those patients off dopamine agonists at the time of treatment. We observed a similar improvement in endocrine remission in patients with acromegaly taken off pituitary-suppressive medications at the time of radiosurgery.82 There remains some controversy, however, as the literature has not been entirely consistent across series.53,69,71,79,87,91 Two other groups analyzed remission rates after radiosurgery in the setting of somatostatin agonists, without an identifiable effect on outcome.69,71 Several issues are postulated to account for this variability. First, the definition of cure is not entirely consistent across series, with some accepting GH values of less than 5 ng/mL.50,72 Second, the rates of follow-up are inconsistent. Pollock et al79 demonstrated that remission continued to occur for up to 5 years following radiosurgical treatment, whereas in the report by Castinetti and colleagues,71 44% of their patient population received the final endocrine evaluation less than 36 months following treatment. Despite inconsistent data, it is the practice at our center to discontinue the use of suppressive medications for 6 to 8 weeks around the period of radiosurgery, with the exact duration contingent on the specific pharmacokinetics of the specific substance used. The rates of biochemical remission vary broadly across series, and one may note from the previously quoted results that a differential sensitivity to radiosurgery has been demonstrated for specific types of secretory pituitary adenomas.17,32,52 In general, Cushing syndrome demonstrates the highest average rates of biochemical remission, followed by acromegaly, prolactinomas, and Nelson syndrome. This finding has been attributed to patient selection, tumor volume, radiation dose delivered, use of suppressive medications, and duration of follow-up.17,52 In an attempt to control for such confounding variables, Pollock et al52 reviewed a retrospective series of 46 patients similar in terms of the above-mentioned aspects. This analysis revealed an 87% remission rate for Cushing disease versus 67% for acromegaly and 18% for prolactinomas (HR, 4.4; 95% CI, 1.1–18.2; P = 0.04). Importantly, although only 18% of patients in this series with prolactinomas met the criteria for remission, the majority (82%) demonstrated symptomatic improvement. Concerning prolactinomas in particular, radiation-induced damage to the pituitary stalk may yield mild elevations of prolactin, resulting in falsely lowered rates of remission.52 Although this supports the hypothesis that radiosensitivity varies with hormonal product, the etiology of this disparity remains obscure. Over all, few cases of recurrence following documented biochemical remission are reported.45,53 However, in these series, rates of up to 20% have been reported. This again serves to accentuate the necessity of long-term radiographic and endocrinologic follow-up after any therapeutic intervention for secretory pituitary adenomas. Adverse events following radiosurgery for a pituitary adenoma remain uncommon, with hypopituitarism occurring the most frequently. On average, 6 to 23% of patients develop some form of anterior pituitary deficiency subsequent to treatment. This has been correlated with the tumor volume; those with a tumor volume of 4.0 cm3 or less have a 5-year risk of 18%, versus 58% for those with larger lesions.29 The incidence of postradiosurgical hypopituitarism is also likely to be related to the preradiosurgical status of the normal pituitary gland, type and timing of prior treatments, radiosurgical dose per volume delivered to the normal gland, and rigorousness and length of the follow-up assessment period. A safe radiosurgical dose or dose per volume below which hypopituitarism will not occur is unlikely to exist. However, a lower dose achieved in part through a steep gradient index is intuitively pleasing in terms of minimizing the risk for hypopituitarism. The second most common side effect includes neuropathies of cranial nerves II, III, IV, V, and VI, which occur in 2% or fewer of all patients. Greater conformality and shielding strategies serve to minimize this risk.92 Other rare side effects include radiation necrosis of the adjacent parenchyma,32,52,53,57 internal carotid artery stenosis,53,93 and secondary tumor formation.94 Concerning radiosurgically induced neoplasia after the treatment of a pituitary tumor, no cases have been reported to date. Nevertheless, one must always be cognizant of this potential, especially when using radiosurgery in younger patients. Meningiomas are common intracranial lesions, and although they are generally histologically benign, resection of skull base lesions can be associated with significant morbidity.95–109 Given the intimate relationship of the sellar and parasellar region to critical neurovascular structures, tumor control and preservation of neurologic function have become the goals of therapy. Previously, external beam radiotherapy was the adjuvant treatment of choice following surgical resection. However, more recently, radiosurgery has replaced this modality and has even become an acceptable primary therapy in certain cases. For WHO grade 1 meningiomas, SRS results in control rates ranging from 86 to 100%,110–138 with a recent series demonstrating 5- and 10-year progression-free survival rates of 95% and 69%, respectively139 (Fig. 14.2; Table 14.6). Variability in tumor and treatment characteristics and the duration of follow-up are all partially responsible for the differential outcomes reported. Specific variables that have demonstrated statistical significance in predicting tumor control include tumor volume and patient age at the time of treatment, with a younger age portending a more favorable outcome. In our experience at the University of Virginia, those lesions larger than 5 cm3 had an average peripheral dose of 12.7 Gy, compared with 15 Gy for smaller tumors (P < 0.001).139 This finding serves as an impetus for cytoreductive surgery before radiosurgical therapy. When the sellar or parasellar meningioma is adjacent to critical, radiation-sensitive structures such as the optic apparatus despite maximal safe resection, multiple-session radiosurgery with LINAC-based systems or the Gamma Knife eXtend may achieve a high rate of tumor control and maintain neurologic function.140 As with pituitary adenomas, WHO grade 1 meningiomas are slowly progressive lesions, and long-term follow-up is necessary to adequately characterize treatment outcomes. There is evidence suggesting that meningiomas followed for longer periods of time will either progress or decrease in size, whereas tumors with shorter follow-up remain constant in size.22,139 For patients with atypical and malignant meningiomas, SRS affords a much lower rate of tumor control.141–144 In one study by Kano and colleagues, a margin dose exceeding 20 Gy to WHO grades 2 and 3 meningiomas was found to yield a higher chance of tumor control.141 In general, SRS, like other treatment tools including resection, radiotherapy, and brachytherapy, offers a reasonable but far from perfect rate of local and distant tumor control for WHO grades 2 and 3 meningiomas. A higher margin dose should be delivered to the tumor during radiosurgery whenever possible. Cranial neuropathies were the most frequently observed adverse event (0–10%). These typically were secondary to tumor progression rather than to radiation-induced damage.110–138 This compares favorably with the reported range of 18 to 41% following microsurgical resection.95–109,145 Factors demonstrating an association with cranial neuropathy were those one would expect to be associated with tumor progression (larger volume, lower peripheral dose, and longer follow-up). In the series by Williams et al,139 only tumor progression demonstrated significance on multivariate analysis, and 79% of patients who developed a new neurologic deficit did so in this setting. Contrary to what was demonstrated for pituitary adenomas, hypopituitarism was an infrequent finding. SRS has come to occupy an important role in the management of patients with parasellar and sellar tumors. Radiosurgery can be used after resection in patients with substantial residual tumor or tumor recurrence. It can also be used as an upfront treatment for some patients. Long-term tumor control is typically afforded by radiosurgery for those with pituitary adenomas and benign meningiomas. Neurologic function is generally preserved or improved, even when a tumor in the cavernous sinus is treated. Delayed tumor progression or radiation-induced complications are rare. However, lifelong follow-up of patients is generally recommended.
 Radiosurgical Devices and Techniques
Radiosurgical Devices and Techniques
 Radiosurgery for Pituitary Adenomas
Radiosurgery for Pituitary Adenomas
Nonfunctioning Pituitary Adenomas
Secretory Pituitary Adenomas
Cushing Syndrome
Nelson Syndrome
Acromegaly
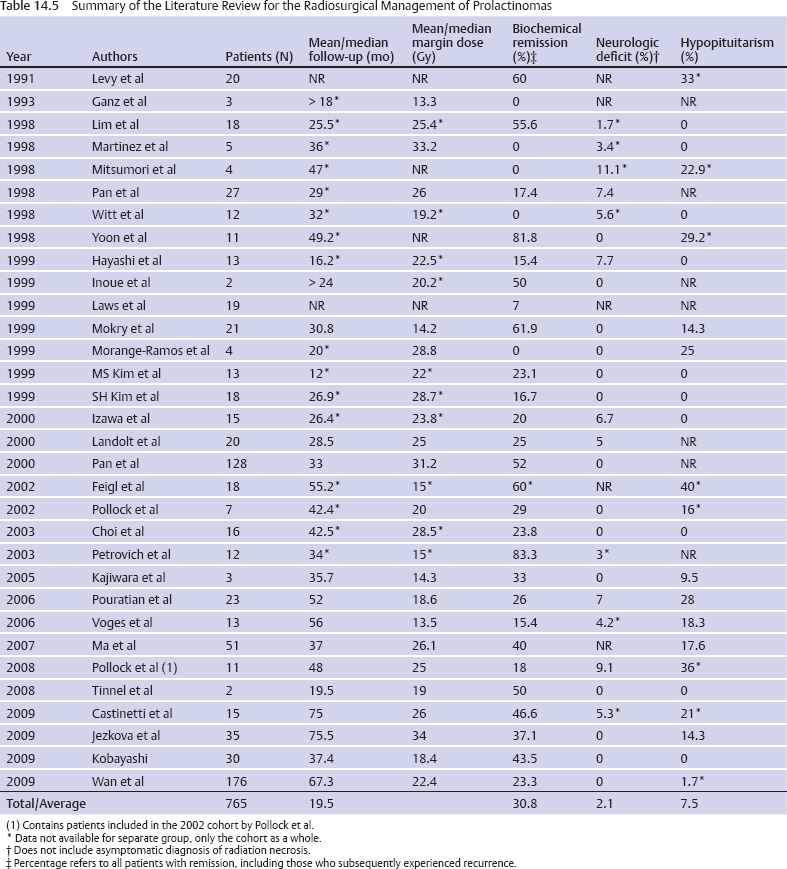
Prolactinoma
Biochemical Remission and Late Recurrence
Adverse Events
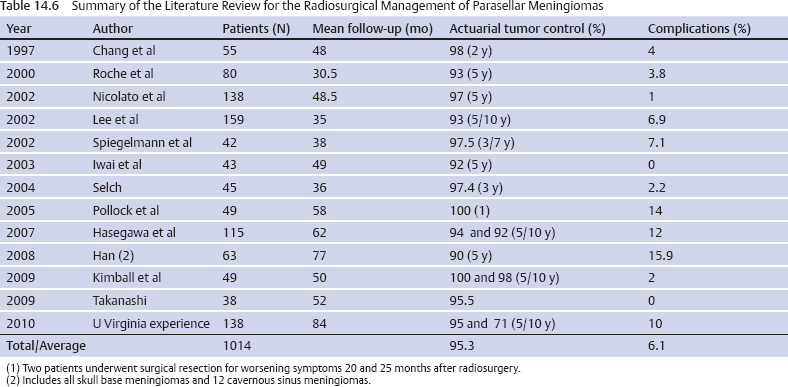
 Radiosurgery for Sellar and Parasellar Meningiomas
Radiosurgery for Sellar and Parasellar Meningiomas
Tumor Control
Adverse Events
 Conclusion
Conclusion
References