Acute brain magnetic resonance imaging diffusion-weighted (a) and apparent diffusion coefficient map (b) images showing evidence of acute ischemic lesions in right frontal cortex and para-ventricular white matter areas.
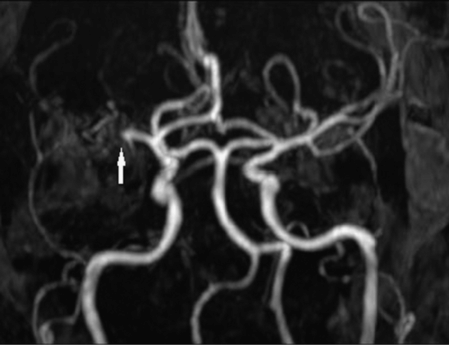
After 5 days she was transferred to our medical center’s cardiology service expressly for consideration of PFO closure. Transesophageal echocardiogram (TEE), with intravenous infusion of agitated saline, confirmed an inter-atrial shunt and visualized a PFO. Hypercoagulable work-up was entirely negative. Repeat imaging with brain CT (not shown) and CT angiogram revealed evolving right hemispheric infarction, without hemorrhagic transformation, and restoration of normal flow in the right MCA (Figure 10.3).
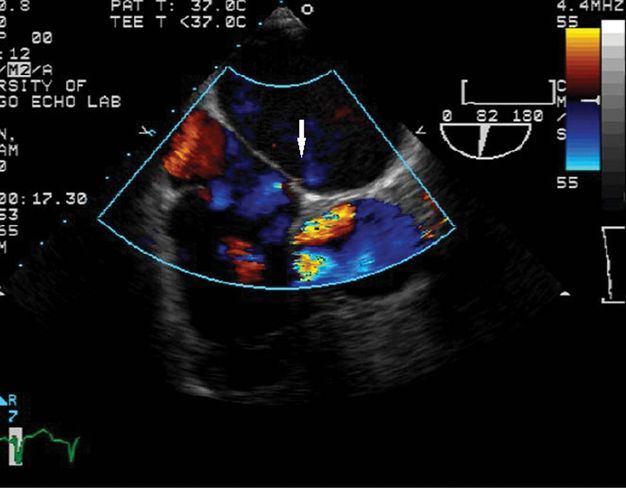
She was treated initially with aspirin and supportive care. Seven weeks later, percutaneous closure was accomplished using a 25 mm Amplatzer cribriform PFO closure device (Figure 10.4). She tolerated the procedure without complication. One day later, transthoracic echocardiography with bubble study showed the device in place at the interatrial septum and “a minimal interatrial shunt.” She was discharged, and was lost to subsequent follow-up at our center.
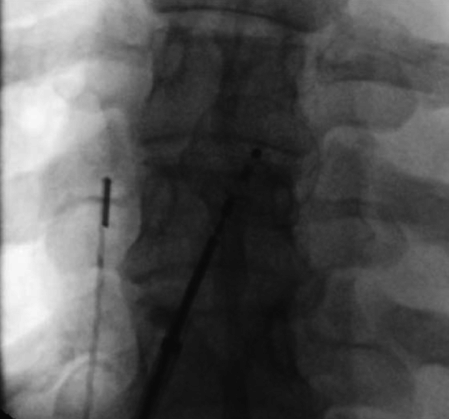
Fluoroscopic image from closure-device placement, showing intracardiac echocardiogram transducer and device delivery catheter, attached to hub of closure device, immediately preceding deployment.
Discussion
Documented proximal MCA occlusion, threatening a devastating stroke, followed by recanalization, as in this case, provides a recognizable pattern highly suggestive of embolic stroke. In a young person with few vascular risk factors, paradoxical embolism rises high on the a priori list of possible etiologies to consider. The findings of a PFO, with atrial septal aneurysm, by Bayesian statistical reasoning, raises this from possible to probable cause, although alternative embolic sources can never be fully ruled out.
It stands to reason that paradoxical embolism must start with venous thromboembolism. The presence of known risks for venous thrombosis, such as smoking and oral contraceptives in this case, are common in patients with paradoxical embolism, even when Doppler examination fails to confirm deep venous thrombosis. A lack of identified venous thrombosis is common even in cases of pulmonary embolism, so that negative findings on lower extremity venous Doppler examination cannot be considered as ruling out paradoxical embolism from a venous source. Some centers advocate additional work-up to include pelvic CT or MRI scanning to search further for venous thrombi, which, when positive, may strengthen the diagnosis of presumed paradoxical embolism. The treatment of such patients must address the risks of recurrent venous thrombosis and pulmonary embolism as well as risks of recurrent stroke.
Best medical therapy for prevention of recurrent stroke in cryptogenic stroke associated with PFO is not well-established. One might rationalize that the supposed etiology of the stroke, due to paradoxical embolism from a venous source, would dictate that anticoagulation must be recommended, as the more effective therapy for prevention of venous thromboembolism. If deep venous thrombosis can be directly identified, then clearly anticoagulation is indicated, at least for 3 months [8]. However, lacking identified venous thrombosis, the balance between risks and benefits of anticoagulation versus antiplatelet therapy may be different. The embolic source might be a superficial vein, or the atrial septum itself. Furthermore, recurrent stroke following cryptogenic stroke in a young person with PFO has been shown to be a relatively low-risk event, substantially less frequent than the early recurrence rate of venous thromboembolism after initial deep venous thrombosis. For long-term prevention of an uncommon event the risk/benefit balance between full anticoagulation and antiplatelet therapy is not the same as that for the short-term prevention of recurrent venous thromboembolism. Thus, many practitioners have chosen to use antiplatelet therapy rather than anticoagulation for secondary prevention in their patients with cryptogenic stroke and PFO.
The finding of atrial septal aneurysm (ASA) with PFO on echocardiography may suggest a higher risk condition than PFO alone. Mas et al. [3] found that PFO with ASA was a marker of increased risk of recurrent stroke in young patients with cryptogenic stroke, associated with a rate of stroke recurrence of 15.2% over 4 years, much more than PFO alone, associated with a 4-year rate of stroke recurrence of 2.3%, not significantly greater than (and numerically less than) the incidence of recurrence in those without either PFO or ASA.
The advisability of recommending PFO device closure for cryptogenic stroke associated with PFO involves all of the issues above, as well as the risks and effectiveness of the devices in preventing recurrent embolization. While randomized controlled trials, as reviewed above, have not successfully demonstrated effectiveness of device closure for stroke prevention in their primary endpoints, in patients with PFO and ASA, in the RESPECT trial [6], there was a strong signal suggesting efficacy of closure over medical treatment. Off-label use of a closure device can be offered on these grounds, with careful explanation to the patient of the lack of conclusive evidence for efficacy and the lack, at the present time, of device approval for stroke prevention.
A confounding factor in decision-making for patients with stroke and PFO is often the influence of the expectations of patients and referring physicians. If told that medical therapy might be a more appropriate course of action for secondary prevention in his/her case, the patient will sometimes respond with the implication that the physician is depriving him or her of the life-saving procedure “to fix the hole in my heart.” While these influences must be tempered with careful patient education about what is known and not known about stroke and PFO, and about the effectiveness of device closure, shared doctor–patient decision-making inevitably brings these expectations into play.
The timing of closure following stroke, if it is to be attempted, should be carefully considered. Prior to the dual antiplatelet therapy that is temporarily required after device placement, hemorrhagic conversion of the stroke must be ruled out. A period of recovery of brain tissue from acute effects of ischemia, and restoration of normal autoregulatory function, should be allowed before challenging the patient with an intracardiac procedure. For infarctions of moderate size or larger, a period of at least 4–6 weeks between stroke occurrence and device placement is appropriate.
Tip
When a patient with PFO has been referred directly to the cardiologist, inevitably the final decision regarding PFO device closure occurs between the patient and the physician offering the procedure. The neurologist serving in a consulting role has the responsibility to provide verification of the nature of the cerebral event as ischemic, to ensure that a complete etiological work-up has been completed and has been interpreted correctly, and to fully inform the patient regarding the implications of the finding of PFO and the uncertainties regarding the best approach to secondary prevention.
Case 2. Transient focal neurological symptoms
Case description
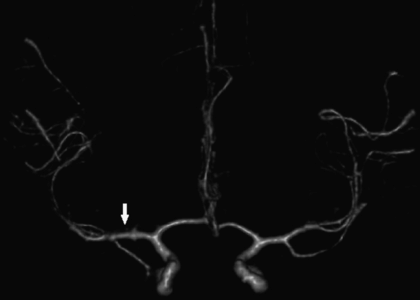
A 31-year-old man awoke from a nap during a long ride back from a holiday with family and experienced right hand, arm, and face tingling and numbness, difficulty reaching with the right hand, and garbled word order in expressive speech. Stopping at a gas station, he felt lightheaded and had trouble reading a billboard. These symptoms abated within 10 minutes, but a bifrontal headache occurred. He was admitted to a local hospital and extensive work-up was done, including brain MRI scanning, cervical spine MRI, head and neck MRA, transcranial Doppler (TCD) study, and MRI of pelvis, and antiphospholipid antibody screening, all entirely normal. However, a TEE was positive for left-to-right flow across the interatrial septum, suggestive of PFO (Figure 10.5, not this patient).
Three weeks later he had another event that was similar, but less severe, with trouble talking on the phone, difficulty finding words, and right arm tingling, lasting minutes, without headache. He was referred to our medical center for evaluation for possible PFO closure. Review of medical history revealed only occasional mild headaches associated with a sick feeling in the stomach, and two episodes of syncope, once while fasting, another time at the sight of blood.
On examination he was a thin fit young man, appearing somewhat anxious. His vital signs were normal, with blood pressure 117/78 mmHg, and his entire neurological examination was normal.
He was advised that these events with apparent aphasia and right sensory disturbance were possible TIAs, with alternative possibilities including migrainous events. Based on the clinical features, with uncertainty regarding the diagnosis of cerebral ischemia, and lack of high-risk PFO anatomical features, closure of PFO was not recommended. Instead, continued antiplatelet therapy, and evaluation for hyperlipidemia and need for statin therapy were advised.
Over subsequent 6 years he had recurrent symptoms that he learned to recognize as anxiety-associated, with symptoms of panic attacks. These were successfully treated by his primary physician with low dose alprazolam as needed.
Discussion
Diagnosis of transient events, without lasting signs and without MRI scan correlates, is inherently less certain than diagnosis of stroke with lasting objective signs. Transient symptoms are difficult to assign to specific etiology. Diagnosis is based entirely upon historical characteristics of the symptoms and associated patient factors. Scoring systems using clinical features to provide a numerical score predictive of risk of recurrent stroke, such as the ABCD2 score, have been validated. The risk stratification they produce is probably based more on classifying transient neurological events in terms of the likelihood of their true thromboembolic nature, rather than finding different risk implications for different kinds of true ischemic events. It is notable that the ABCD2 score [9] does not award any points for purely sensory symptoms. The features of this case, with primarily sensory symptoms, short duration, and no accompanying vascular risk factors, would produce a low ABCD2 score, casting doubt on the ischemic nature of the event. Furthermore, recurrent events with stereotypical symptoms are not consistent with a cardiac source of emboli, as flow in the heart is turbulent and emboli from the heart will travel disparate paths. While two successive symptomatic emboli might with some probability go to the same MCA territory, more than two events with similar symptoms should point to a non-embolic cause or intracranial or possibly cervical source of emboli. Finally, the associated headache raises the possibility of migraine as cause. Reports of palpitations or panic might point to anxiety-driven symptoms.
Given these challenges in accurate diagnosis of etiology of transient events, it is notable that TIA was included as an entry criterion for the CLOSURE I study, likely contributing some patients with non-embolic causes for symptoms to the study pool, and decreasing the study’s power to detect the effectiveness of device closure for preventing recurrent stroke.
The presence of a PFO in a patient with an event of uncertain nature is not unexpected by chance alone as an incidental finding. An urge to reach premature diagnostic closure, assigning blame for the event to the PFO, must be resisted. The lack of high-risk anatomical features of the PFO in this case, without right-to-left shunt at rest, or associated ASA, are further reasons to discourage an interventional procedure. In other patients, the reason for discovery of a PFO might be the work-up done for a stroke not likely to be cardioembolic in nature, such as a lacunar infarction in a middle-aged patient with hypertension or diabetes, or a stroke in the territory of an artery with documented atherosclerotic plaque. Such patients should not be subjected to PFO device closure.
Tip
In patients with substantial doubts regarding the diagnosis of a cerebral embolic ischemic event, unproven therapies with substantial risk such as endovascular PFO closure or prolonged anticoagulation should be regarded with skepticism. In such cases, the neurologist’s role needs to be one of providing clarity of thinking about the etiology of the event, redirecting the focus away from the incidental finding of a PFO and towards the more pertinent risk factors.
Case 3. Cryptogenic stroke with PFO and thrombophilia
Case description
A 30-year-old right-handed woman, with history of hypertension, smoking, preeclampsia, asthma, and spontaneous abortion, and known to carry the factor V Leiden mutation (heterozygous), developed sudden chest pain and dyspnea. At her local hospital, chest CT angiogram was equivocal for pulmonary embolism, and she was not anticoagulated. Three months later, she again experienced sudden severe chest pain, followed 5 minutes later by right-sided numbness involving face, arm, leg, and trunk. The right hand was clumsy and the speech was slurred, with word-finding difficulties. Most symptoms and signs improved within 2 hours, and brain CT and MRI scans were reportedly negative. Repeated chest CT scan showed no sign of pulmonary embolism. Carotid duplex scanning showed no stenosis or disease. Transthoracic echocardiogram showed entirely normal findings. One week later a repeat echocardiogram was performed, this time with infusion of intravenous bubble contrast, and was reportedly entirely normal, without any detection of PFO or right-to-left shunting. She was diagnosed with TIA and released without further treatment. She stopped smoking.
Her physician referred her for a neurovascular opinion. Aspirin was recommended immediately, as well as consideration of long-term anticoagulation for venous thromboembolism prophylaxis, based on clinical suspicion. To further search for paradoxical embolism, TCD study with intravenous bubble infusion was performed. This revealed prominent passage of bubbles into the bilateral MCAs at rest, and markedly increased shunting with Valsalva maneuver (Figure 10.6). Based on this finding, a recommendation was made for secondary stroke prevention with either long-term anticoagulation for venous thromboembolism prophylaxis, or device closure of the PFO. The question of whether anticoagulation might also be indicated for prevention of venous thromboembolism was referred to her internist.
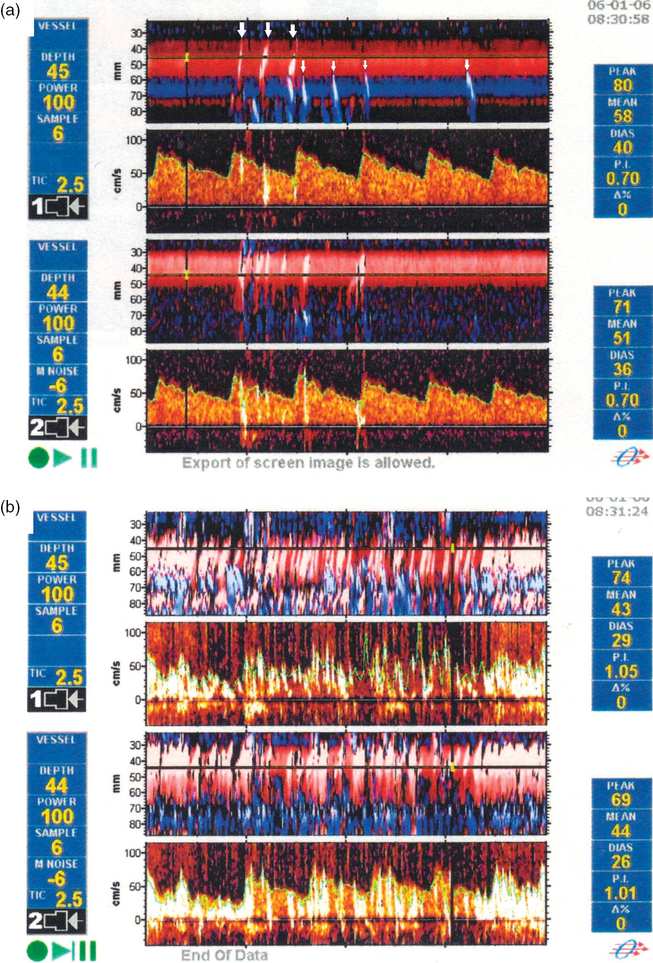
Frame shots from TCD recordings during venous infusion of agitated saline bubbles, from a patient with similar findings to those of this case. (a) At rest, frequent individual high-intensity transient signals at depths consistent with MCA or ACA localization are seen (arrows). (b) With a 5-second Valsalva maneuver, the device screen is filled with multiple overlapping high-intensity transient signals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








