Radiology: Cranial ultrasound has low sensitivity for detecting AIS; unilateral decreases in MCA flow velocity may be seen. Computed tomography (CT) is insensitive for venous thrombosis and early AIS, but relatively quick and sensitive for hemorrhagic lesions. Magnetic resonance imaging (MRI) is modality of choice for arterial stroke in the newborn; diffusion-weighted imaging (DWI) can reliably detect ischemic injury within 24 h of onset (Fig. 18.1), but may pseudonormalize within 7 d (although at this stage T1- and T2-weighted imaging should be abnormal). Encephalomalacia and calvarial hypertrophy (Dyke-Davidoff-Masson phenomenon) can be seen as late sequelae of perinatal stroke (Fig. 18.2). Magnetic resonance angiography (MRA) of the neck should be obtained if there is a history of traumatic delivery or other reason to suspect neck trauma resulting in injury/dissection of cervicocephalic vessels. Echocardiography should be performed to exclude congenital heart defects and intramural thrombi. Doppler ultrasound of the umbilical vessels and portal vein should be performed if umbilical catheters are present.
Laboratory: Initial laboratory evaluation should include hematocrit and prothrombotic risk factors: activated protein C resistance for factor V Leiden, protein C, protein S, lipoprotein(a), antithrombin III, prothrombin gene mutation, homocysteine/MTHFR gene mutation, antiphospholipid antibodies. Repeat testing in 6 to 8 wk is recommended for protein C, protein S, antithrombin III, lipoprotein(a), and antiphospholipid antibodies.6
Placental pathology: Often informative due to presence of placental infarcts, vasculopathy, or evidence of perinatal infection.
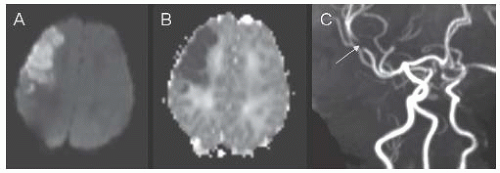 FIGURE 18.1 Corresponding axial slices from diffusion-weighted imaging (DWI) (A) and apparent diffusion coefficient (ADC) (B) MR sequences demonstrating a right frontal arterial ischemic infarction in a 2-day-old boy who developed focal seizures at 29 h of life. The MR angiogram (C) shows focal occlusion of the anterior division of the right middle cerebral artery (arrow). |
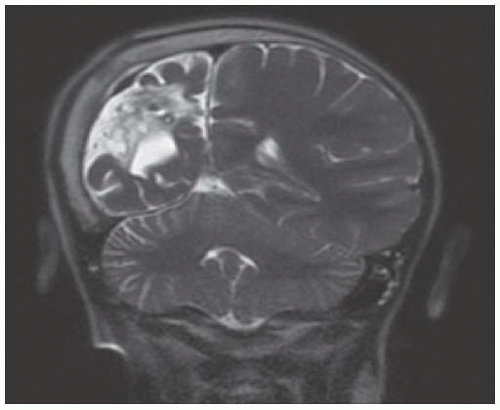 FIGURE 18.2 Coronal T2-Weighted MR Image from an 8-Year-Old Boy with Presumed Perinatal Arterial Ischemic Infarct Affecting the Right Middle Cerebral Artery. The image demonstrates encephalomalacia and atrophy of the right hemisphere, with overlying calvarial hypertrophy (Dyke-Davidoff-Masson phenomenon). |
period occur in 6% to 20%. CVT with infarction is associated with worse neurodevelopmental outcomes than CVT without infarction. Recurrence risk is thought to be low, although data are limited.15,16
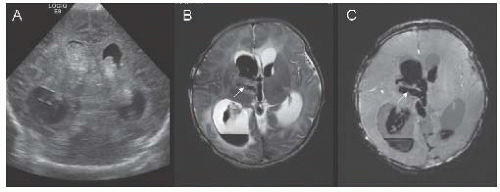 FIGURE 18.3 Coronal transfontanel head ultrasound image (A) from a 1-day-old boy with seizures, showing bilateral intraventricular hemorrhage and associated ventriculomegaly. Axial T2-weighted (B) and susceptibility-weighted (C) MR images illustrate thrombosis of the right internal cerebral vein (arrows). |
TABLE 18.1 Recommended Evaluation for Children with Arterial Ischemic Stroke | ||||||
|---|---|---|---|---|---|---|
|
Supportive measures include avoidance of hypoxemia and hyperthermia, maintaining normal blood glucose. Thrombolytic therapy (tPA) for acute stroke is currently not recommended in children due to lack of evidence, but is sometimes used in older adolescents who meet adult tPA eligibility criteria.
Short-term anticoagulation may be initiated in children until the cause of stroke is determined and continued for up to 1 wk. Low molecular weight heparin: 1 mg/kg every 12 h; monitor anti-factor Xa activity drawn 4 to 6 h after dose (goal 0.5-1 U/mL). Unfractionated heparin may also be used, especially in situations where need for reversal of anticoagulation is anticipated.
Long-term anticoagulation is indicated in children with risk of recurrent cardiac embolism, arterial dissection, and certain hypercoagulable states. Low molecular weight heparin usually initial choice; may be continued or transitioned to warfarin or newer oral anticoagulants (e.g., rivaroxaban).
Antiplatelet agents may be used for secondary prevention of strokes in children without SCD who are not known to have high recurrent risk of embolism or severe hypercoagulability. Aspirin is usually dosed at 3 to 5 mg/kg/d; if dose-related side effects occur, may reduce to 1 to 3 mg/kg/d. Clopidogrel 1 mg/kg/d has been used in children unable to tolerate aspirin.
If homocysteine is elevated, it is reasonable to supplement with folate, B12, or pyridoxine. Oral contraceptives should be discontinued in children with ischemic stroke.
Between 50% and 80% of surviving children have neurologic sequelae, most commonly hemiparesis; other problems include poor attention, behavioral problems, and cognitive deficits. Poorer outcome is associated with systemic disease, multiple risk factors, infarct size, cortical involvement, thromboembolism, and moyamoya.1
CT/MRI may show small areas of infarction in cortical watershed zones, basal ganglia, deep white matter, or periventricular regions. MRI may show absence of flow voids in ICA, MCA, and ACA with abnormal flow voids from basal ganglia and thalamic collaterals (Fig. 18.4).
EEG shows slowing of the background rhythm after hyperventilation (“re-buildup”).
TCD may assist with diagnosis and postoperative follow-up.
Various techniques used to determine resting perfusion/blood flow reserve and to predict improvement in functional perfusion after therapy (xenon CT, PET, MR perfusion, acetazolamide SPECT).
Screening (with MRA) may be considered in individuals with common high risk-associated conditions or prior history of unilateral disease (27% eventually develop bilateral involvement).
Surgical revascularization (by direct or indirect anastomoses) is used in patients with cognitive decline or recurrent or progressive symptoms.25 Meta-analyses suggest that 87% derive symptomatic benefit from revascularization, but no differences between types of procedures.29 Potential complications: postoperative stroke, spontaneous or traumatic subdural hematoma, ICH. Perioperative management includes sedation and analgesia (to prevent crying and hyperventilation), avoidance of hypotension, hypovolemia, hyperthermia, and hypocarbia.
Antiplatelet therapy (aspirin 3-5 mg/kg) may be used when patient has a poor operative risk or has mild disease, or routinely after surgery.
Calcium channel blockers may improve intractable headaches and reduce frequency and severity of refractory TIAs.
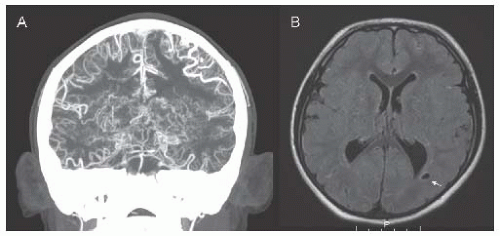 FIGURE 18.4 CTA (A) and FLAIR MRI (B) in an 8-year-old boy with trisomy 21 and moyamoya syndrome. CTA shows terminal stenosis of the bilateral internal carotid arteries distal to the origin of the posterior cerebral arteries, with exuberant deep collaterals (moyamoya vessels) as well as pial collaterals. MRI shows chronic white matter infarcts (arrow) in the posterior-middle cerebral artery border zones. |
TABLE 18.2 Nonneurologic Manifestations of Fabry Disease | |||
|---|---|---|---|
|









