Stroke in Children
Sally M. Sultan
INTRODUCTION
There are two classifications for pediatric stroke: by age and by stroke subtype. By age, a perinatal stroke occurs during gestation through 28 days of life. Often, a child is discovered to have a chronic infarct in infancy that is presumed to have occurred during the perinatal period. These are referred to as presumed perinatal strokes. A stroke that occurs after 28 days through 18 years is a childhood stroke. Childhood stroke can be further subdivided into infancy (older than 28 days to younger than 2 years), childhood (2 to 11 years), and adolescence (12 to 18 years). By subtype, pediatric stroke is subdivided as arterial ischemic stroke (AIS), cerebral venous thrombosis (CVT), and hemorrhagic stroke. Hemorrhagic stroke includes intracranial hemorrhage (ICH), intraventricular hemorrhage (IVH), and subarachnoid hemorrhage (SAH). This chapter will begin with a discussion of stroke subtypes, will continue by looking at the unique features of stroke related to sickle cell anemia (SCA) and perinatal and presumed perinatal stroke, and will conclude with a brief review of genetics of pediatric stroke.
ARTERIAL ISCHEMIC STROKE
EPIDEMIOLOGY
Annual incidence for childhood AIS is 1.2 to 7.9 per 100,000 children. After the neonatal period, pediatric AIS peak among school-aged children. From the first 676 patients in the International Pediatric Stroke Study (IPSS) registry, the largest registry of all pediatric stroke, the median age at first AIS presentation was 5.7 years (interquartile range, 1.7 to 11.6 years).
For all childhood stroke, there is a predominance of affected males. In the California discharge database, the relative risk (RR) for ischemic and hemorrhagic stroke was higher among boys than girls: for AIS, RR 1.25 (confidence interval [CI] 1.11 to 1.40); SAH, RR 1.24 (CI 1.00 to 1.53); and ICH, RR 1.34 (CI 1.16 to 1.56). Mortality after AIS was also higher among boys (17% vs. 12%). The ratio of boys-to-girls is generally estimated to be 6:4. IPSS found that 59% of children with AIS are male.
Recent studies in the United States are reporting racial and ethnic disparities in childhood stroke where black children are at higher risk for ischemic and hemorrhagic stroke than white children. Pediatric stroke mortality was found to be higher in stroke belt states (southeastern United States), compared with other U.S. states.
PATHOBIOLOGY
Arteriopathy (50% of Arterial Ischemic Stroke)
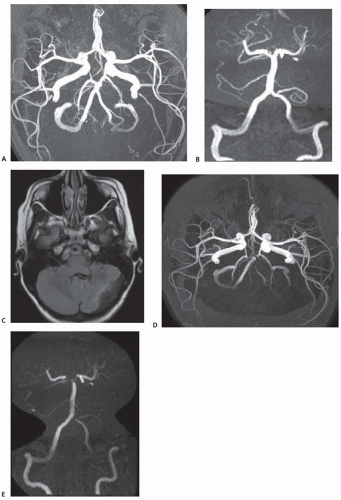
Arteriopathy in pediatric stroke is presumed to be nonatheromatous and instead due to inflammation, infection, and inherited disorders of tissue stability and repair. Arteriopathy is found in approximately 50% of childhood AIS, and discrete radiographic and clinical subtypes have begun to be described. Two reported predictors of arteriopathy are a recent upper respiratory infection and SCA. The traditional nomenclature for AIS arteriopathy subtypes are focal cerebral arteriopathy (FCA), moyamoya, intracranial and extracranial arterial dissection, vasculitis, sickle cell arteriopathy, postvaricella arteriopathy, and other unspecified forms of arteriopathy (Fig. 144.1). (Frequencies of arteriopathy subtypes are as reported in the IPSS registry.)
FCA (25% of arteriopathy). FCA is typically characterized by unilateral mainstem arterial disease with lenticulostriate infarction. An East Asian study found that FCA was reversible in 68%, progressive in 20%, and stable in 12%. Of the patients with reversible arteriopathy, 53% had early worsening. In European cohorts, the course of unilateral arteriopathy was progressive in only 6%, stabilized in 32%, improved in 45%, and completely normalized in 23%. Among patients with a nonprogressive arteriopathy, 44% had an antecedent varicella infection, and 18% had a recurrent stroke or transient ischemic attack (TIA).
Moyamoya (22% of arteriopathy). Moyamoya arteriopathy is the progressive development of bilateral occlusive disease of the terminal internal carotid arteries (ICAs) with basal collaterals (see also Chapter 43). There may also be involvement of the mainstem branches of the terminal ICA. Moyamoya syndrome is used when there is an accompanying condition known to be associated with moyamoya arteriopathy and moyamoya disease when the disease is idiopathic. Moyamoya arteriopathy is commonly progressive. In a single-center series, progression developed in 45% over 5.4 ± 3.8 years. Stroke recurrence is also high from 20% over 4 years to 38% over 10 years.
Craniocervical arterial dissection (CCAD) (20% of arteriopathy). CCAD in childhood includes intracranial and extracranial dissections. In a meta-analysis of arterial dissection in children younger than 18 years, males were affected considerably more than females: 74% male in anterior circulation dissections and 87% male in posterior circulation dissections. This predominance persisted even for nontraumatic dissections. Of dissection involving the anterior circulation, 40% were extracranial and of dissections involving the posterior circulation, 79% were extracranial. One-third of dissections are spontaneous, but less than a fifth are associated with significant trauma. The mean age of presentation is older than all AIS at 8 to 10 years. Common presenting symptoms are hemiparesis and headache. Neck pain was less common in children. Follow-up after an average of 5.2 years showed moderate or severe deficits in 14%, recurrent strokes in 9%, and recurrent dissections only after anterior circulation dissection and in 10% of those patients.
Vasculitis (12% of arteriopathy). Cerebral vasculitis is rare and can be difficult to diagnose in children (see also Chapter 42). Vasculitis due to systemic inflammatory conditions, viral, fungal, mycobacterial, and even parasitic infections is more likely than primary central nervous system (CNS) angiitis.
Consider vasculitis where there are infarcts in multiple vascular territories with microhemorrhages.
Sickle cell arteriopathy (8% of arteriopathy). (See later discussion on SCA, and Chapter 118)
Post-varicella angiopathy (7% of arteriopathy) traditionally stenosis of the distal internal carotid and mainstem branches with lenticulostriate infarction. (See later discussion on “Risk Factors, Infection.”)
Cardiogenic (30% of Arterial Ischemic Stroke)
Cardiogenic causes of stroke include congenital heart disease, valvular heart disease, arrhythmias, cardiomyopathy, cardiac surgery and catheterization, extracorporeal membrane oxygenation, and Kawasaki disease.
Congenital heart disease is the most common cardiac risk factor, reported in 59% of those with a cardiogenic stroke. An isolated patent foramen ovale (PFO) was reported in 15% and cardiac catheterization in 8%.
Thromboembolic (15% of Arterial Ischemic Stroke)
Thromboembolic stroke may be due to an indwelling catheter, infection, or a hypercoagulable state that is inherited or acquired. (See “Risk Factors, Thrombophilia Traits.”)
Cryptogenic Stroke
Cryptogenic stroke is reported in approximately one-fifth of children.
RISK FACTORS FOR ARTERIAL ISCHEMIC STROKE
Infection (25% of Arterial Ischemic Stroke)
The earliest reports suggesting an association between infection and stroke are in children. Stroke as a complication of bacterial meningitis was thought to be due to inflammation of the vessels traversing infected meninges involving perforating and pial arteries. Subsequently, large-artery involvement and recovery-phase large-artery stenosis was recognized.
Varicella-zoster virus (VZV) was the first viral infection associated with cerebral vasculopathy and ischemia after it was described in a 5-year-old boy following a primary VZV infection. Among young children with AIS, a VZV infection in the preceding year was at least three times more frequent compared with published population rates and controls. On imaging, vascular stenosis in the proximal portion of major arteries was seen in 20 of 22 children with AIS and VZV in the preceding year.
Recently, minor infection is found in association with AIS. In the northern Californian Kaiser Pediatric Stroke Study (1993 to 2003), a minor infection (acute fever, upper respiratory tract infection, pneumonia, acute otitis media, pharyngitis, urinary tract infection, or acute gastroenteritis) independently predicted stroke risk in the month that followed the infection. Similarly, chronic inflammatory conditions such as autoimmune disease were highly associated with stroke risk.
Acute Head and Neck Disorders (25% of Arterial Ischemic Stroke)
Acute head and neck disorders include head trauma, pharyngitis, meningitis, intracranial surgery, otitis media, sinusitis, and mastoiditis.
Acute Systemic Conditions (20% of Arterial Ischemic Stroke)
Acute systemic conditions includes fever longer than 48 hours, sepsis, shock, dehydration, acidosis, and anoxia.
Chronic Systemic Conditions (20% of Arterial Ischemic Stroke)
Chronic systemic conditions include SCA, indwelling catheter, genetic disorders, malignancy, and connective tissue disorders.
Chronic Head and Neck Disorders (10% of Arterial Ischemic Stroke)
Chronic head and neck disorders most commonly refer to migraine, brain tumor, and ventriculoperitoneal (VP) shunt.
Thrombophilia Traits
Most thrombophilic states contribute only a small component to an individual’s risk for stroke, but these tendencies are prevalent in the population and may be more important with other risk factors in children with stroke. The largest systematic review to date of thrombophilia risk factors in childhood, AIS and CVT found that in children with first AIS compared with healthy children, there was a significantly greater odds of having an MTHFR C677T, factor V G1691A, or factor II G20210A mutation; antithrombin deficiency; protein C deficiency; protein S deficiency; elevated lipoprotein(a); and antiphospholipid antibodies. The American Heart Association Stroke Council suggests that it is reasonable to test for the more common prothrombotic states even when another stroke risk factor has been identified (Class IIa, Level of Evidence C).
CLINICAL FEATURES
The most common symptoms of an AIS in children is hemiparesis and a speech disturbance. In a single-center pediatric stroke registry, 22% of children with acute AIS presented with seizures. Similarly, an inpatient database in Taiwan found that early symptomatic seizures occurred in 26% of children with AIS.
DIAGNOSIS
Imaging
Patients who present with new focal neurologic deficits should undergo an urgent computed tomography (CT) scan of the head to rule out acute hemorrhage. Early ischemic stroke may not be seen on head CT and should be confirmed with magnetic resonance imaging (MRI). In children with moyamoya disease, transcranial Doppler (TCD) may be useful to follow for stability or progression of vascular disease. (Class IIb, Level of Evidence C).
Stroke Classifications
A new classification system for childhood AIS was recently proposed, the Childhood AIS Standardized Classification and Diagnostic Evaluation (CASCADE) criteria. The system is divided into acute classifications with arteriopathy subdivided by location: small-vessel arteriopathy of childhood, unilateral FCA of childhood, bilateral cerebral arteriopathy of childhood, aortic/cervical arteriopathy, cardioembolic, other, and multifactorial and chronic classifications: progressive, stable, reversible, and indeterminate arteriopathy. These classifications are not yet evidence-based.
TREATMENT
Acute care after AIS begins with maintenance of normal oxygenation, normothermia, normoglycemia, and control of systemic hypertension (Class I, Level of Evidence C). It is reasonable to treat dehydration and anemia (Class IIa, Level of Evidence C). There is no evidence that in children with normal oxygenation, supplemental oxygen provides benefit, and in children without clinical or electrographic seizures, prophylactic administration of antiepileptic medications is not necessary (Class III, Level of Evidence C). There is no data confirming the safety and efficacy of hypothermia as an acute treatment for stroke (Class III, Level of Evidence C). The use of thrombolysis (tissue plasminogen activator [tPA]) is not recommended outside research protocols (Grade 1B). When treatable stroke risk factors are discovered, those conditions should be treated (Class I, Level of Evidence C).
Initial therapy in children without SCA should be unfractionated heparin (UFH) or low molecular weight heparin (LMWH) or aspirin (1 to 5 mg/kg/day) until dissection and embolic causes have been excluded. Once excluded, daily aspirin prophylaxis (1 to 5 mg/kg/day) for a minimum of 2 years should be continued (Grade 1B). In children receiving aspirin who have recurrent AIS or TIAs, consider changing to clopidogrel or an anticoagulant (Grade 2C).
Craniocervical Arterial Dissection
The American Heart Association Stroke Council recommends beginning treatment of an extracranial CCAD with UFH or LMWH and to continue subcutaneous LMWH or bridge to warfarin for 3 to 6 months of treatment (Class IIa, Level of Evidence C). The American College of Chest Physicians recommends anticoagulant therapy for at least 6 weeks, with ongoing treatment dependent on radiologic assessment (Grade 2C). One can also consider using an antiplatelet agent instead of LMWH or warfarin. Beyond 6 months, for recurrent symptoms, it is reasonable to continue anticoagulation or an antiplatelet agent. If symptoms have resolved but there is residual abnormality of the dissected artery on imaging, it is also reasonable to continue an antiplatelet agent beyond 6 months (Class IIa, Level of Evidence C). Surgical procedures may be considered for symptoms due to CCAD despite optimal medical therapy (Class IIb, Level of Evidence C). For an intracranial dissection and for SAH resulting from CCAD, anticoagulation is not recommended (Class III, Level of Evidence C).
Moyamoya Arteriopathy
With moyamoya disease, surgical revascularization can effectively reduce the risk of stroke (Class I, Level of Evidence B). Children with moyamoya should be referred to an appropriate center for consideration of revascularization (Grade 1B). There is less literature to recommend one surgical procedure over another, but indirect revascularization may be preferred in younger children whose smallcaliber vessels make direct anastomosis difficult, and direct bypass techniques may be preferable in older individuals (Class I, Level of Evidence C). Indications for revascularization surgery include progressive ischemic symptoms, evidence of inadequate perfusion, or collateral reserve (Class I, Level of Evidence B). After revascularization surgery or in asymptomatic individuals with moyamoya disease, aspirin may be considered (Class IIb, Level of Evidence C). In general, anticoagulants are not recommended with moyamoya disease because of the risk of hemorrhage and the difficulty of maintaining therapeutic levels in children (Class III, Level of Evidence C).
Supportive care in children with moyamoya disease includes techniques to reduce anxiety and pain that could lead to hyperventilation-induced vasoconstriction (Class IIb, Level of Evidence C). Perioperative and intraoperative avoidance of hypotension, hypovolemia, hyperthermia, and hypocarbia may reduce the risk of perioperative stroke (Class IIb, Level of Evidence C).
Cardiogenic Stroke
Treatment for congestive heart failure and congenital heart lesions is recommended and may reduce the likelihood of cardiogenic embolism. This includes resection of an atrial myxoma and does not yet apply to PFOs (Class I, Level of Evidence C). It is reasonable to begin anticoagulation following a cardiac embolism in children who are judged to have a high risk for recurrence. UFH or LMWH can be introduced as a bridge to warfarin (Class IIa, Level of Evidence B). LMWH can be continued instead of warfarin, and it is reasonable to continue either LMWH or warfarin for at least 1 year or until the lesion responsible for the risk has been corrected (Class IIa, Level of Evidence C). For a suspected cardiac embolism with an unknown or lower risk of stroke and that is unrelated to a PFO, it is reasonable to treat with aspirin for at least 1 year (Class IIa, Level of Evidence C). It is reasonable to close large atrial septal defects (excludes PFOs) to prevent stroke (Class IIa, Level of Evidence C).
In patients with endocarditis of a prosthetic valve, it may be reasonable to continue long-term anticoagulation (Class IIb, Level of Evidence C). Anticoagulation should not be used for native valve endocarditis, and it is not necessary to remove a cardiac rhabdomyoma in an individual who has been asymptomatic (Class III, Level of Evidence C).
Inherited Thrombophilic State
In children with AIS or CVT, it is reasonable to measure the serum homocysteine level and to introduce therapies such as diet and supplementation of folate, vitamin B6, or vitamin B12 to lower elevated homocysteine values (Class IIa, Level of Evidence B). In an adolescent with AIS or CVT, it is reasonable to discontinue oral contraceptives (Class IIa, Level of Evidence C).
Other Causes
Children with Fabry disease should receive α-galactosidase replacement therapy (Class I, Level of ‘Evidence B).
REHABILITATION
A child who has suffered a stroke should be enrolled in ageappropriate rehabilitation and therapy program (Class I, Level of Evidence C). Cognitive and behavioral performance is especially important in school-aged children. The pediatric stroke outcome measure (PSOM) is based on a detailed age-appropriate, neurologic exam and scored for four domains: sensorimotor deficit, language deficit— production (excludes dysarthria), language deficit—comprehension, and cognitive or behavioral deficit. The weight of language and cognitive and behavioral deficits is considerably greater and motor deficits are less weighty compared with similar adult stroke outcome measures. Neuropsychological assessment is useful for planning therapy and educational programs and monitoring cognitive, behavioral, and language deficits after a stroke (Class I, Level of Evidence C).
OUTCOME
Neurologic Deficits
In one single-center series of 90 children followed for a mean of 2.1 years after an AIS, 49% were either normal or mildly impaired by PSOM.
Recurrence
In northern California, the 5-year cumulative recurrence rate was 19% after childhood AIS. All of the recurrences occurred among children with a vascular abnormality. In a large single-center series, 37% of children had a recurrent AIS or TIA at a median of 267 days (range 1 day to 11.5 years). Of the 84% of patients who had reimaging, 11% had clinically silent recurrent ischemia. Among children with arteriopathy in northern California, the 5-year cumulative recurrence rate was 66%. Factors associated with silent reinfarction and clinical recurrences were moyamoya, low birth weight, genetic thrombophilia, previous TIA, bilateral infarction, preexisting condition, and leukocytosis.
Epilepsy
Poststroke seizures and epilepsy is considerable in children. A population-based retrospective study of children with AIS found an average annual incidence rate of first unprovoked seizure of 4.4% (95% CI, 3.3 to 5.8), 16% (95% CI, 12 to 21) at 5 years, and 33% (95% CI, 23 to 46) at 10 years. The risk of epilepsy was 13% (95% CI, 9 to 18) at 5 years and 30% (95% CI, 20 to 44) at 10 years. An inpatient database in Taiwan found epilepsy in 17% of children after AIS.
CEREBRAL VENOUS THROMBOSIS
EPIDEMIOLOGY
Population-based incidence studies report that less than 1 per 100,000 children per year are diagnosed with CVT (see also Chapter 40). Many stroke providers believe true incidence is significantly higher and diagnoses are missed. Among patients with a presumed diagnosis of idiopathic intracranial hypertension (IIH), routine imaging discloses CVT in as many as 26%. CVT also peaks in school-aged children. The mean age of children at the time of diagnosis is 5 to 9 years.
PATHOBIOLOGY
The cerebral veins and sinuses are thin-walled and without valves. Cerebral veins drain into the sinuses that form from dural pockets around the brain. Unlike arterial vascular territories, thrombosis of particular veins does not present with well-defined clinical syndromes. The venous system has considerable anatomic variability, flow can be reversed, and there may be collateral drainage pathways such as through occipital sinuses.
The cerebral veins and sinuses are positioned for thrombotic complications. Venous drainage is slow; it follows a tortuous route and the walls easily distend. A combination of local inflammation in the vessel wall, systemic thrombophilia, hyperosmolality, reduced blood flow, and injury to the vessel wall all work to tip the balance toward cerebral thrombosis. When a thrombus obstructs venous outflow, the pressure becomes high in the venous system. Edema and hemorrhage can develop from high pressure in the capillaries with plasma and red blood cell diapedesis or frank rupture, and the high pressure can exceed arterial perfusion pressure resulting in ischemic infarction. Hydrocephalus with intracranial hypertension will occur when cerebrospinal fluid reabsorption through arachnoid villi and pacchionian body granulations in the dural sinuses becomes impaired. A large thrombus in the superior sagittal sinus, the sinus where most of the reabsorption occurs, will generally lead to a communicating hydrocephalus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree