Chapter 45 Suboccipital Retrosigmoid Surgical Approach for Vestibular Schwannoma (Acoustic Neuroma)
For the surgery of vestibular schwannoma (acoustic neuroma), refinements of microsurgical techniques, combined with improvements in intraoperative monitoring of facial and cochlear nerve function and advances in neuroimaging, have resulted in the shift of focus from reduction of mortality to optimizing facial nerve function,1–4 hearing preservation,1,2,5 and preservation of other cranial nerves.6 Facial nerve dysfunction is a major concern among patients undergoing unilateral vestibular schwannoma surgery, and loss of useful hearing can be debilitating.7 Patients with vestibular schwannoma have many options, including watchful waiting, radiosurgery, fractionated radiation, and surgery through one of several routes: translabyrinthine, middle fossa, or suboccipital retrosigmoid. The ideal treatment strategy depends on several factors, including the age, medical condition, and hearing status of the patient and the anatomy of the tumor as seen on imaging studies. Surgeons generally try to save hearing if the speech discrimination is above 50% and the average pure tone loss is less than 50 dB.8,9 However, even worse hearing is worth saving in some circumstances, such as when poor hearing is present on the contralateral side or in a patient with neurofibromatosis type 2 (NF2), where bilateral hearing loss could later occur. Moreover, these various treatments are not mutually exclusive; in some patients, a combination of subtotal surgical tumor removal followed by radiosurgery of the residual may prove appropriate.
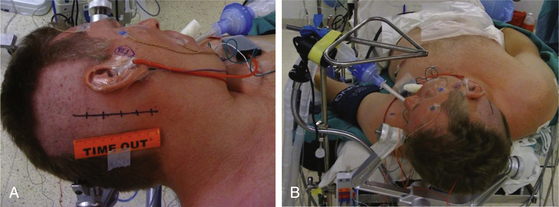
The patient is positioned supine with one or two folded soft cotton blankets beneath the ipsilateral shoulder and padding beneath the knees and is secured with one or two belts to allow the table to be rolled if necessary during surgery. For patients with a cervical tumor (as in NF2) or elderly patients with cervical spondylosis or limitations in neck movement, additional blankets are used to further turn the upper body of the patient and thereby require less turning of the neck. However, too much turning of the body can place the shoulder in a position that interferes with surgery. To prevent peripheral neural compression, the surgeon must ensure the contralateral ulnar and fibular head areas will not be under pressure if the bed is rolled and pad them appropriately. The head is gently turned toward the contralateral side. Hair is shaved from about 7 cm behind the ipsilateral ear, and the patient is fixed in a three-pin headrest (Fig. 45-1A). The surgeon must assure that there is adequate space between the chin and the clavicle and that contralateral jugular compression is avoided. Physiologic monitoring equipment is placed. Bipolar facial monitoring electrodes are routinely placed in the orbicularis oculi and orbicularis oris. If trigeminal nerve function is of concern, an additional electrode is placed in the masseter. For hearing monitoring, the ear canal is inspected and cleaned if necessary; an electronic clicker is placed in the ipsilateral ear and the contralateral ear, with electrodes in the scalp to record a brain stem auditory evoked response (BAER) during surgery. If lower cranial nerves are involved, we use an endotracheal tube with an embedded electrode.
The self-retaining retractor post is secured to the side of the headrest away from the surgical site. A metal triangle is used to protect the face and allow both the anesthesiologist and the neurophysiologist or audiologist visualization of the airway and of facial movement. The endotracheal tube exits the mouth on the contralateral side to avoid distortion of the ipsilateral lip and cheek. A fiberoptic tube delivers a cool but bright light to the area under the drapes and assures that the small TV camera also under the drapes has a well-illuminated view of the patient’s ipsilateral face (Fig. 45-1B). Stimulation of the facial nerve in the operative field causes a clearly visible “smile” reaction, which can be seen on the external TV monitors. In contrast, electric activity that causes stimulation of the motor branch of the trigeminal nerve in turn causes jaw movements due to activation of the masseter muscle. An audiologist or neurophysiologist monitors facial movements, electromyogram (EMG), and BAER throughout the procedure.
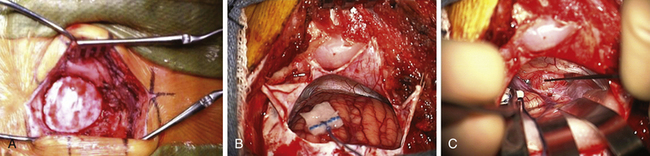
The area behind the operative ear and an area on the ipsilateral abdomen are sterilely prepared and draped for surgery. A linear incision is made approximately 3 cm behind the insertion of the pinna and going from approximately 1 cm above the tip of the pinna to 1 cm below the ear lobe. Superiorly, a 3-cm piece of pericranium is removed and placed in sterile solution during the procedure for later use in closing the dural defect. The muscles are then divided inferiorly, hemostasis is obtained, and two muscle self-retaining retractors are placed. They are angled such that the medial skin area is free from these muscle retractors, because that is where the cerebellar retractors will later be situated (Fig. 45-2A).
The incisional area is surrounded with antibiotic-soaked sponges and towels in preparation for dural opening. The dura is opened with a linear vertical incision approximately 2 cm medial to the edge of the sigmoid sinus. The medial dura is left intact to protect the cerebellum. Additional dural incisions are made laterally toward the edge of the venous sinuses. The resulting triangular-shaped dural leaves are sutured to the surrounding tissue with 4-0 Vicryl sutures. An adjustable bar is attached to the retractor bar that previously had been affixed to the headrest, and a long flexible arm and long narrow retractor are attached. The inferior cerebellum is gently elevated with the retractor, and the arachnoid of the cistern is opened sharply to release CSF and provide for cerebellar relaxation. For larger tumors, a ventriculostomy catheter is put into the cistern, secured to the inferomedial dura with a 4-0 Vicryl suture, cut off at the dural edge, left in place for continuous CSF drainage throughout the procedure, and removed during closure. For smaller tumors, this is often unnecessary. At the time of initial dural opening, if the cerebellum is under a lot of pressure, it is often beneficial to open initially the most inferior triangular dural flap only and then release a small amount of CSF to allow the remainder of the dural opening to be performed under more controlled conditions. The surgeon must, however, be cautious not to remove too much CSF when the dura is not fully opened; doing so can be associated with too much cerebellar relaxation, causing a venous tear near the tentorium or in the area of the petrosal sinus. This can be difficult to control and forces a more rapid dural opening than might be desired.
Once the dura is opened and CSF removed, the cerebellum is generally relaxed and, in this position, falls away from the cerebellopontine angle because of gravity (Fig. 45-2B). A piece of moistened rubber dam covered with Telfa is placed on the cerebellum. Three long flexible arms and three long, 1⁄4-inch-wide self-retaining retractors are used. The retractors are placed on the Telfa with the tips on the cerebellum 1 or 2 mm proximal to the tumor edge. Care must be taken to be sure the retractors do not occlude important vascular structures or impinge on or stretch cranial nerves. They should be formed to sit securely on the skin edge to prevent unwanted movement. Occasionally, with a large tumor and an operculum of cerebellum overhanging the tumor, it is safer to resect the lateral cerebellar operculum than to retract it for long periods.
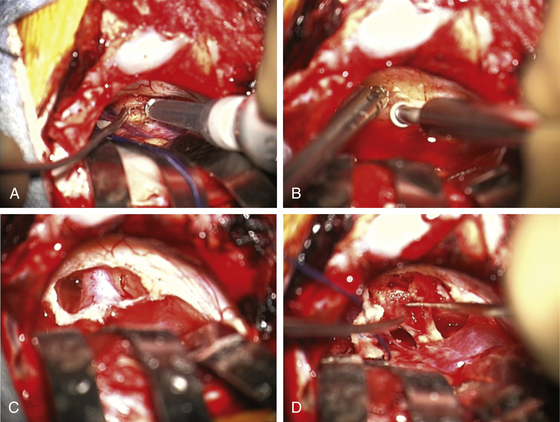
The operating microscope is engaged. At this point, stimulation of the posterior capsule of the tumor is necessary (Fig. 45-2C). I use a monopolar stimulator and first check it at 3 mA on the exposed neck muscles to be sure the stimulator is working and the patient is not pharmaceutically paralyzed. If muscle stimulation occurs, the stimulator is turned down to 1 mA and the posterior capsule is stimulated at all points to exclude a posteriorly placed facial nerve. If no stimulation is encountered, the tumor may be entered. In more than 80% of patients, the facial nerve is anterior or inferior. However, if stimulation occurs, the surgeon must determine whether this is due to transmission to a distant facial nerve or whether the nerve is indeed on the posterior tumor surface. To determine this, the posterior capsule is mapped out at sequentially lower milliampere levels to determine the course of the facial nerve. The surgeon must define an entry point into the tumor that is devoid of facial nerve fibers.
The surgeon must pay close attention to the arachnoid over the tumor. If this can be stripped away without coagulation, it provides not only the best plane for dissection but also a protective barrier for the cranial nerves and their small feeding vessels. After peeling away the arachnoid, a nonstimulating area on the posterior tumor is coagulated with bipolar cautery and opened with microscissors and specimens are sent for pathologic study. The internal portion of the tumor is then decompressed using an ultrasonic aspirator (Fig. 45-3A). Hemostasis is obtained, and the tumor capsule is dissected from the arachnoid plane and rolled inward. Depending on the size of the tumor, this process of internal decompression followed by dissection and inward rolling the capsule may need to be repeated several times. Each time, some of the capsule is also cut away but always leaving a visible portion to make the next round of microdissection easier. The retractors may need to be moved, but the surgeon must always keep them low profile, affixed to the skin, and with the tips on the cerebellum, not the cerebellar peduncle, brain stem, or critical blood vessels.
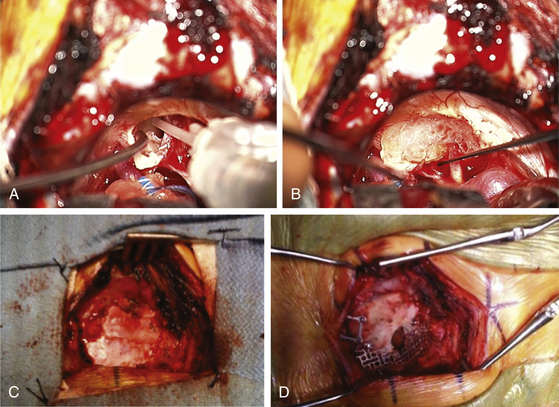
At some point, the limiting factor becomes the entry of the tumor into the internal auditory canal and its attachments at the medial edge of the canal. It then becomes necessary to open the canal. I usually work with an otologist to do this part. However, in some circumstances, the neurosurgeon may do this portion of the procedure. The superior and inferior lips of the internal auditory canal are defined. If there are open CSF cisterns, they are occluded with moistened gelfoam pledgets to prevent entry of bone dust. However, all patties with strings on them must be removed from the field prior to using a drill. The dura over the canal is coagulated and flapped medially. Using a suction-irrigator and high-speed diamond drill (Fig. 45-3B), the canal is opened to expose the dura of the canal (Fig. 45-3C). Attention is paid to air cells that will later need to be secured (Fig. 45-3C) and to a high jugular bulb if present. If hearing is being spared, the drilling of the canal should be limited to 10 mm or less and must not include the vestibular apparatus. Once drilling is completed, the entire operative field is washed clear of bone dust, the temporary gelfoam pledgets are removed, and the CSF spaces are irrigated with saline. The dura of the canal is then opened sharply.
For a case in which hearing is being spared, we do not necessarily define the facial nerve at this point in the canal; rather, because these are smaller tumors, we proceed to define the facial nerve medially as it enters the brain stem. The tumor can then be rolled medially to laterally, sparing both the facial and the cochlear nerves (Fig. 45-3D). There is often a nice arachnoid plane, and as the tumor is rolled into the canal, parts of the tumor may need to be removed to decrease the bulk. During the dissection of tumor off the facial nerve or cochlear nerves, the surgeon must minimize the use of bipolar cautery. If bleeding starts between the tumor and the facial nerve, it can often be stopped with irrigation or temporary application of Surgicel. Even using bipolar cautery at low levels can cause facial or cochlear nerve damage. Ultimately, the most lateral portion of the tumor can be removed with microdissectors. Ideally, the surgeon desires not only that the facial nerve be anatomically intact but also to stimulate at low milliamperes at the brain stem level and that a good wave V remains on the BAER.
Once the tumor is removed and hemostasis is obtained, the internal auditory canal that has been drilled must be secured to prevent CSF leakage postoperatively. A 1-inch incision is made in the skin of the abdomen that had been previously prepared sterilely. A piece of subcutaneous adipose tissue is removed, hemostasis is obtained, and the incision is closed with 3-0 Vicryl sutures in the subcutaneous tissue plus a 4-0 Vicryl subcuticular closure and Steri-Strips. If large air cells are visible where the internal auditory canal had been drilled, some Tisseel or fibrin glue is placed within (Fig. 45-4A) and then packed with one or a few small pieces of adipose tissue covered with bone wax. The drilled surface of the canal is secured with bone wax, and a larger piece or multiple pieces of adipose tissue are then placed over the bone wax. This is covered with one piece of moistened surgicel to secure it to the surrounding dura (Fig. 45-4B). This is then further sealed and secured to the surrounding dura with Tisseel or fibrin glue.
< div class='tao-gold-member'>