The pathology of abnormalities at the craniocervical junction is extensive. A complete knowledge of the bony anatomy, embryology, and biomechanics of the craniocervical junction is necessary to understand the etiology of the abnormalities in this area and, thus, to plan their treatment. Congenital, developmental, and acquired lesions arise at the craniovertebral junction to produce changes that ultimately affect the neural structures. The mainstay of decompression of the posterior fossa and upper cervical spine has been by the dorsal route, at times with a fusion; however, the morbidity and mortality of such procedures, especially when the indication is a ventrally situated abnormality, are high. Operative procedures based on an understanding of the surgical pathology of this region have been developed and are outlined in Table 9–1. Congenital abnormalities at the craniocervical border are now being recognized with increasing frequency at birth and require a careful understanding of the patho-physiology to undertake management. Congenital torticollis as a result of atlas assimilation and rotary subluxation of the atlantoaxial articulation are not uncommon. Similarly, syndromic disturbances, such as the Goldenhar’s syndrome, Klippel-Feil syndrome, fetal warfarin syndrome, and Conradi’s syndrome, are but a few examples that may be associated with craniocervical abnormalities and cervicomedullary dysfunction. The diagnostic imaging of these infants is the same as that of the young child and the adolescent: plain radiographs, computed tomography (CT), and magnetic resonance (MR) imaging. The factors that influence specific treatment are (1) the reducibility of the lesion, the most important factor, which implies restoration of anatomic relationships of the craniospinal axis using position as well as traction; (2) the mechanics of compression and the direction of encroachment; (3) the etiology of the lesion, such as syrinx, Chiari malformation, vascular abnormalities, or basilar invagination; and (4) the presence of epiphyseal growth plates and ossification centers in congenital abnormalities. Figure 9–1 outlines the decision tree for treatment of craniocervical abnormalities. In the neonate as well as the young infant younger than the age of 2 years, craniocervical immobilization is the mainstay of treatment and is accomplished with custom-built orthoses that are changed at 3-month intervals. At the age of 2 years, the child is reassessed for surgical management. Indications for halo traction in the pediatric population are (1) instability and correction of alignment; (2) stabilization during surgery for the cervical spine and the craniovertebral junction; (3) immobilization of the craniocervical region and cervical and upper thoracic spine; and (4) prevention of potential instability. Contraindications for halo-ring placement are (1) age, because it should be avoided in infants younger than 2 years old; (2) scalp infections; (3) inexperience of the surgeon; (4) the presence of bone-softening syndromes; and (5) cardiovascular or respiratory abnormalities or infants prone to seizures.
PREOPERATIVE EVALUATION
Halo-Ring Placement
Approaches for decompression Ventral Midline Transoral-transpharyngeal Transpalatal-transseptal Lateral rhinotomy LeForte I with maxillary “down fracture” Lateral Lateral transcervical extrapharyngeal Preauricular infratemporal Lateral Lateral transcervical with translocation of vertebral artery and partial resection of occipital condyle Dorsal Midline posterior fossa and upper cervical posterolateral decompression Fusions Atlantoaxial Interlaminar Modified Gallie/Brooks Transarticular C-2-C-1 screw fixation Occipitocervical Dorsal interlaminar Titanium loop Plates with screws or cables |
Before placement of the halo ring, the occipitofrontal circumference must be measured and an appropriate pediatric crown halo obtained. It is important that the pediatric pins be allowed to fixate perpendicular to the cranium and at least a 2- to 2.5-cm space be available between the halo ring and the scalp for cleansing as well as for ease of placement of the halo-immobilizing vest. It is important to assess the skull thickness by age as well as by obtaining a head CT scan. The halo ring is placed with the patient under intravenous sedation or general anesthesia. If the latter is used, a cervical collar must be placed around the neck to correct the instability, and paralyzing agents should be avoided. The head is placed on a positioning board that also supports the upper trunk. The crown halo is placed over the cranium, ensuring that it is slightly below the cranial equator. Positioning pins are fixated temporarily with suction cups to the scalp to maintain the halo ring temporarily in place. This allows cleansing of the scalp with 10% PVP-iodine and local infiltration of the pin sites. In children younger than the age of 4 years, 8 to 10 pediatric pins are used. In patients 4 to 8 years of age, 6 pins are recommended. Four pins can be used in patients older than 8 years. These consist of one frontal and one parieto-occipitotemporal pin on each side. These pins are screwed in place, and iodine ointment is placed at the “business” end of the pin; the pin is advanced to touch the scalp. All pins must be brought to position on the scalp before being tightened. Tightening is done with diagonal pins tightened to the recommended pressures. Pressures recommended below in children less than 2 years of age are that which is generated between the thumb and index finger, usually about 1 to 1½ pounds of torque pressure. In patients 2 to 4 years of age, 2 pounds of torque pressure is recommended and 4 pounds in those between the ages of 4 and 6 years. In children between the ages of 6 and 10 years, 4 to 6 pounds of torque pressure is used. The pins are tightened, and a hex nut that accompanies the halo pin is locked into position. Again, at the end of 48 hours, this position is rechecked with the patient under sedation. A bucket handle is attached to the crown halo and placed in a neutral position. The traction that I use in the operating theater is 2 to 3 pounds for a child at 2 years of age and 3 to 4 pounds of traction pressure in a patient between the ages of 2 and 4 years.
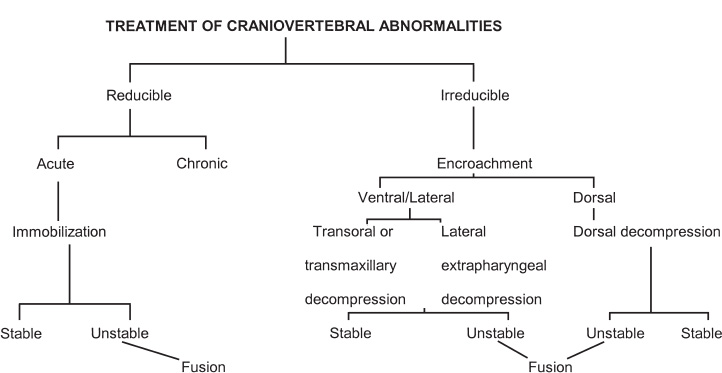
FIGURE 9–1. Treatment of craniovertebral abnormalities.
It is extremely essential that halo-ring traction placement in the pediatric population be only carried out by an experienced team comprising a pediatric neurosurgeon/orthopaedic surgeon, radiologist, intensive care nursing staff, and physicians conversant in the problems mentioned. Cervical spine radiographs must document the effects of the traction immediately as well as at close intervals, at times on a daily basis.
TRANSORAL–TRANSPALATOPHARYNGEAL DECOMPRESSION OF THE CRANIOCERVICAL JUNCTION
Indications
The transoral–transpalatopharyngeal approach to the craniovertebral junction is the most frequently used route for ventral decompression of the cervicomedullary junction. It is indicated only in non-reducible ventral extradural osseous compression (Fig. 9–2) of the cervicomedullary junction and at times with extradural tumors, such as chordoma and osteoblastoma. It is contraindicated in most cases of intradural pathology or in extradural pathology that is more than 15 mm from the midline.
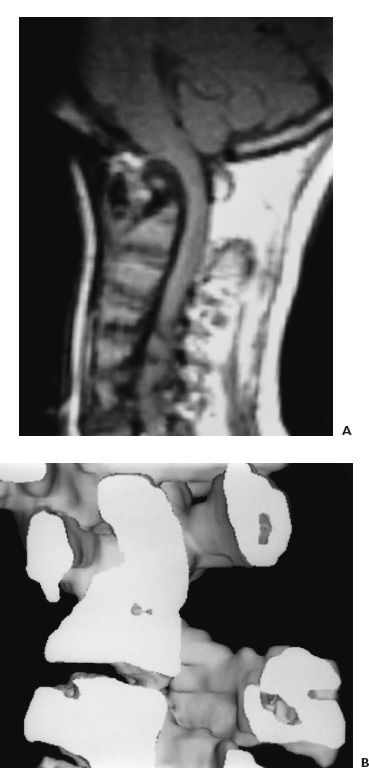
FIGURE 9–2. A: Midsagittal T1-weighted MR image of the craniocervical junction in a 10-year-old boy with fixed atlantoaxial dislocation causing severe ventral cervicomedullary compression. B: Three-dimensional CT scan of the upper cervical spine and craniocervical junction with midsagittal visualization of the fixed atlantoaxial dislocation and severe compromise of the spinal canal.
Oropharyngeal cultures are obtained 3 days before a surgical procedure. No antibiotics are given unless pathological flora are present. If the lower cranial nerves are compromised, it is imperative that preoperative assessment of the respiratory function as well as swallowing mechanism be made to determine whether a tracheostomy is needed at the time of the transoral operation or immediately thereafter.
Intraoperative Technique
The child is transported to the operating theater in skeleton traction on a fracture bed with an MR imaging compatible halo at 5 to 6 pounds of traction in the older child in whom preoperative traction is possible. In the young child, the halo ring is applied after general anesthesia is obtained. This is achieved with a cervical collar in place and mask induction, followed by fiberoptic oral endotracheal intubation made through the mask; subsequently, the halo ring is applied. Nasal endotracheal intubation is avoided because it disrupts the integrity of the high nasopharyngeal mucosa, which is the avenue of approach to the craniocervical border. The head then rests on a padded Mayfield horseshoe headrest; traction is maintained over a pulley bar at the weights recommended. In an older child, that is, between the ages of 12 and 16 years, an awake intubation is made, and the patient is positioned. The awake patient is examined to ensure that no neurologic change has occurred during positioning, after which general endotracheal anesthesia ensues. In the last 8 years in our institution, a tracheostomy has not been performed in a child or an adult undergoing transoral–transpalatopharyngeal approach to the craniocervical junction. Following intubation, a gauze packing is placed to occlude the laryngopharynx and to prevent secretions and blood from draining into the stomach.
A modified Dingman self-retaining mouth retractor secures the endotracheal tube and allows exposure of the oral cavity as well as the pharynx (Fig. 9–3A). In procedures that involve the foramen magnum and clivus, it is essential to split the soft palate to provide the necessary exposure.
The operating microscope provides magnification and a concentrated light source. The oral cavity is cleansed with 10% povidone iodine and hydrogen peroxide and then rinsed with saline. A midline incision is made into the soft palate extending from the hard palate to the base of the uvula and deviating to one side. Stay sutures are applied to the incised soft palate, retracting the flaps to either side exposing the high posterior nasopharynx down to the C-3 vertebral level (Fig. 9–3B). The posterior pharyngeal wall is first topically anesthetized with 2.5% cocaine and the median raphe with 0.5% lidocaine solution with 1:200,000 epinephrine.
A midline posterior pharyngeal incision is made from the midclivus to the upper border of C-3. The pharyngeal flaps are retracted to either side with stay sutures, and the longus colli and longus capitis muscles are dissected free of their osseous-ligamentous attachment to expose the lower clivus, the anterior arch of C-1, and the body of C-2. Lateral exposure is limited to 1.5 cm to either side of the midline in a child to preserve integrity of the hypoglossal nerve superiorly, the eustachian tube orifice, and the vertebral arteries in descending order.
For an inexperienced surgeon working in the transoral area, it is extremely important to recognize that congenital abnormalities with a foreshortened clivus mandate that there is platybasia and that the clivus is far from the hard palate. This situation necessitates removal of the caudal hard palate to achieve exposure with the clivus.
The anterior arch of the atlas and the caudal clivus are removed using a high-speed drill. The soft tissue ventral to the odontoid process is resected with rongeurs (Fig. 9–3C). The odontoid process then is removed in a rostral caudal dimension using the diamond burr; the pannus then is excised (Fig. 9–3D).
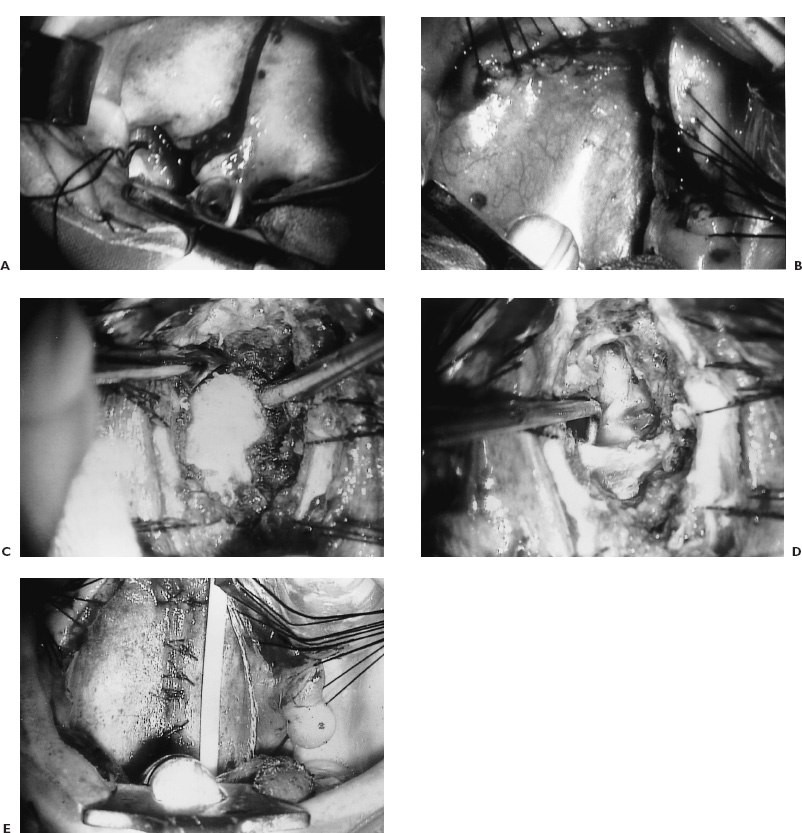
FIGURE 9–3. A: Operative photograph through the microscope with Dingman mouth retractor in place. The endotracheal tube and the tongue are depressed with the tongue blade. The cheek retractors improve the illumination and visualization. The incision in the soft palate starts at the hard palate and proceeds to the base of the uvula and skirts to one side. B: The leaves of the soft palate are held apart with stay sutures to expose the mucosa of the high nasopharynx. C: The posterior pharyngeal musculature as well as the longus colli and longus capitis muscles are swept to either side of the midline to expose the odontoid process after the anterior arch of the atlas has been resected. Note the apex of the odontoid process here. D: The odontoid process has been resected and a right angle separator identifies the transverse portion of the cruciate ligament. E: The longus colli and longus capitis muscles have been approximated as is the posterior pharyngeal wall. A nasogastric feeding tube is visualized.
Pannus signifies instability. It should be resected but not beyond the limits of the exposure. Bipolar cauterization will allow shrinkage. Division of the odontoid process at its base and downward traction should be avoided. It is tempting to proceed with this; however, the retained fragments of odontoid tip are testimony to inadequate operative procedure and an inexperienced surgeon. Depending on the bulging of the dura into the wound, the tectorial membrane may or may not be divided. The cruciate ligament usually is visualized at the caudal aspect of the bony exposure. It is wise to leave the transverse portion of the cruciate ligament intact for partial stability of the atlas.
If a tumor such as chordoma is encountered, direct visualization of its extent is needed before piecemeal resection is done. Undue traction must be avoided because a pedicle onto the vertebral artery or a major branch may be present.
Resection of an intradural lesion requires relieving cerebrospinal fluid turgidity by having previously placed a lumbar subarachnoid drain. The dura is opened in a midline fashion, extending up as high as necessary into the ventral posterior fossa. This cruciate dural incision is made by converting the vertical incision to a cruciform one below the foramen magnum, thus avoiding the circular sinus. Closure demands that an attempt be made to bring together the leaves of the dura in a watertight fashion insofar as possible. If the dura has been violated, it is closed and backed with fascia harvested from the external oblique aponeurosis, after which a fat pad is placed before the posterior pharyngeal wall is closed.
The longus colli and longus capitis muscles are approximated in the midline with subsequent anatomic layered closure of the posterior pharyngeal musculature and the posterior pharyngeal mucosa. I use 3–0 polyglycolic suture. A soft feeding tube is inserted through the nose into the stomach and allows feeding in the early postoperative phase (Fig. 9–3E).
Closure of the soft palate is done by bringing the nasal mucosa together with interrupted sutures, and subsequently the muscularis and the oral mucosa are approximated with a through-and-through interrupted vertical mattress suture of similar strength. The patient is maintained in a Philadelphia collar and turned over for the fusion procedure, most often carried out using the same anesthetic (Fig. 9–4B).
Postoperative Management
No oral intake is permitted for the first 5 days. Nasogastric tube feedings are initiated on the day after surgery and are followed by a gradual increase in feeding until a regular diet is resumed 15 to 18 days after the surgery. If the dura was opened, intravenous antibiotics (cefotaxime, metronidazole and methicillin) are administered and spinal drainage maintained for 10 days after the operation. The endotracheal tube is removed only after lingual swelling has receded, usually after an average of 3 to 4 days. Table 9–2 outlines the possible complications of this procedure and the treatments.
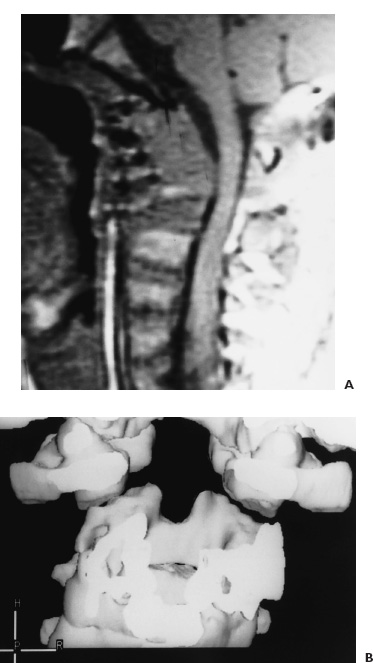
FIGURE 9–4. A: Postoperative T1-weighted MR image of the craniocervical junction demonstrates the ventral osseous decompression of the cervicomedullary junction. B: Three-dimensional CT scan of the craniocervical junction in the frontal plane reveals the resection of the odontoid process into the midportion of the axis body.
LATERAL TRANSCERVICAL EXTRAPHARYNGEAL APPROACH TO THE CRANIOCERVICAL JUNCTION
Indications
This approach has been of interest to surgeons who fear contamination of the oral cavity. The advantage is the ability to proceed with a ventral fusion if this is necessary and also the potential for placing C-2 to C-1 lateral mass screws. This procedure is difficult, however, because it goes into a narrowed pyramid at the apex with restriction of exposure.
| Complication | Prevention and Management |
| Perioperative complications | |
| Unnecessary transoral procedure | Preoperative reduction if possible |
| Damage to eustachian tubes and hypoglossal nerves | Limit lateral exposure to 2 cm from midline |
| To o small an oral exposure | May need to split mandible and tongue |
| Inability to reach clivus due to platybasia | Divide soft palate and possibly hard palate; intraoperative fluoroscopy |
| Lost! cannot reach or resect odontoid or epidural masses | Fluoroscopy; start resection at the rostral end |
| Persistent bleeding | Pannus and arterial bleeding must be controlled with bipolar coagulation; bleeding from circular sinus needs Avitene and Oxycel; else clip both leaves of dura |
| CSF leakage; intra-arachnoid lesion | Preoperative lumbar drain; attempt dural closure; fascial graft and fat pad and plasma glue; CSF drainage X 1 wk. Triple antibiotics X 10 days |
| Delayed complications | |
| Severe tongue swelling | Decadron; intermittent release of tongue depression; retain dental guards in children |
| Meningitis | CSF examination and lumbar drainage; no oral intake; antibiotics; close leak |
| Palatal dehiscence | Immediate reclosure |
| Pharyngeal dehiscence | <1 wk – reclosure; >1 wk hyperalimentation and antibiotics |
| Neurological worsening | Check alignment – traction: meningitis; abscess? retained lesion? vascular compromise? |
| Retropharyngeal abscess | Check for osteomyelitis and meningitis; extrapharyngeal drainage |
| Delayed pharyngeal bleeding | Secondary infection; rule out osteomyelitis and vertebral artery erosion with false aneurysm; MRI and angiography |
| Velopalatine incompetence | Usually appears 4–6 mo postoperatively; pharyngeal retraining; may need pharyngeal flap |
CSF, cerebrospinal fluid; IV, intravenously; MRI, magnetic resonance imaging.
There is a high risk to injury of the hypoglossal and the glossopharyngeal nerves and difficulty in visualizing deep midline structures of the true craniocervical border. Likewise, the clivus is poorly visualized using this approach.
Intraoperative Technique
The awake patient undergoes fiberoptic intubation through a nasal endotracheal route. The oral cavity is kept free of any tubes. The same precautions are taken as with the transoral route regarding anesthesia and intubation.
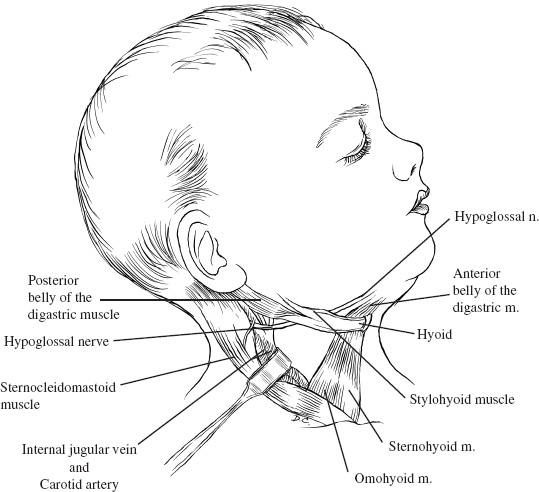
The cervical incision starts behind the ear, proceeds over the mastoid process, and extends 1.5 cm below the angle of the mandible toward the midline above the hyoid bone. An inferior extension of this at the level of the sternomastoid muscle converts the transverse incision into a T. The head is turned to the left in the case of a right-handed surgeon. The extent of the vertical limb of the incision depends on the amount of cervical spine that needs to be exposed. The incision traverses through the subcutaneous tissue and the platysma. Subcutaneous dissection allows mobilization in a subplatysmal plane of the superficial fascia. The inferior division of the facial nerve is identified, and facial nerve dissection into the parotid gland may be needed for mobilization. The superficial draining veins into the jugular vein are dissected free and ligated prior to their entrance into the common facial vein or the jugular vein. Superficial branches of the facial nerve are protected by staying deep to this vein.
The deep fascia at the anterior border of the sternomastoid muscle is incised allowing for visualization of the carotid sheath. Dissection is made anterior to the vascular structures when the midline and the opposite side of the craniocervical junction are necessary. The sub-mandibular salivary gland may be elevated. Resection of the submandibular salivary gland has no consequences if its duct is sutured properly to prevent a salivary fistula. The nodes in the carotid and the digastric triangle are removed. The posterior belly of the digastric is traced to its tendon, where it is transected and transfixed with a suture for subsequent reapproximation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


