TREMOR
Tremor, of different etiologies, can be a very disabling symptom when becomes refractory to medical treatment. In such circumstances, surgical therapies should be advised. Surgical therapies include stereotactic and γ-knife thalamotomies, and deep brain stimulation (DBS) of the thalamus. The efficacy of DBS to treat severe tremor has been shown to be comparable to thalamotomy; however, thalamic stimulation is associated to fewer adverse effects and results in a greater functional improvement (1). DBS has essentially replaced lesion procedures, due to its efficacy, safety, and reversibility (2). In developed countries, classical ablative surgery has become obsolete and abandoned in the treatment of tremor.
ESSENTIAL TREMOR
Essential tremor (ET) is the most common movement disorder. It is defined as a 4- to 12-Hz postural and kinetic tremor involving the arms, hands, or fingers but sometimes involving the voice, the head, or other body parts during voluntary actions such as eating and writing. The estimated prevalence of ET is 0.4% to 3.9%, with even higher prevalence (4.6%) in people over 65 years of age (3). Some patients can suffer severe tremor interfering with the performance of many activities of daily living; in such cases where medical treatments fail DBS can be considered. Since thalamic DBS has established its efficacy in the treatment of ET, all patients with medically refractory tremor are potential candidates to receive thalamic DBS; elderly patients should be advised about increased DBS-related surgical risks. The most generally recognized brain target is the anterior margin of the ventral intermediate (VIM) nucleus of the thalamus.
Measured through standardized scales, substantial (40%–85%) and generally sustained during long-term follow-up improvement has been found after VIM-DBS in overall ET symptoms. Besides, important improvements in hand function, handwriting, and activities of daily living have been reported (4–12), as well as improvement in quality of life (6,12–16). Some of the studies showing the efficacy of VIM-DBS in the control of symptoms in ET have been designed with the use of blinded assessments (4,5,13,17,18).
The effect of VIM-DBS specifically on head-tremor symptoms has been established in two studies (5,19), with bilateral VIM-DBS being more effective than unilateral stimulation in one study (19). Voice-tremor outcomes in ET patients after VIM-DBS stimulation seem to be somewhat mixed. According to one study including seven ET patients, voice tremor improved significantly only in patients who had severe symptoms, and there were no notable differences between patients who underwent unilateral versus bilateral VIM-DBS (20). Significant improvement in voice tremor (83%) in patients undergoing bilateral stimulation compared to unilateral stimulation was observed in another study (19), whereas no improvement in voice tremor was noted in 19 ET patients (12 unilateral, 7 bilateral) postoperatively and at 6 years after VIM-DBS stimulation in another (7).
The most common adverse events associated with this therapy include paresthesias, dysarthria, and disequilibrium. These side effects are typically mild and generally amenable to changes in stimulation parameters. One of the studies has reported the overall hardware-related complication rate to be 23.5% (11). Few studies report no overall change in cognitive functioning following VIM-DBS. One study looking at cognitive outcomes in ET patients at 1 year reported no overall deleterious effects of unilateral VIM-DBS on cognition (6).
Recently, new surgical techniques have been applied to treat medically refractory ET. The combination of high-resolution MRI and improved focused ultrasound transducer technology has led to the emergence of the field of MR-guided focused ultrasound surgery (MRg-FUS). This technique has appeared as a potential noninvasive alternative for the treatment of tremor. This system combines a clinical 3-T MRI system with a transcranial hemispheric array transducer (650 kHz) that has 1,024 ultrasound elements. The procedure consists of a series of low-power sonications producing temperatures of 40°C to 45°C given in order to confirm accurate focusing by means of MR thermography. Final sonications produce temperatures between 55°C and 65°C. Tissue heating can be brought beyond the threshold for protein denaturation with resulting necrosis depending on a predictable temperature–time relationship. In a recent pilot study (21), unilateral MR-guided focused ultrasound in the VIM provided effective tremor relief at 3 months in four patients with ET. Patients had a reduction of tremor in their targeted arm of about 80% at 3 months. However, one of the patients presented paresthesias persisting at 3 months and another suffered a deep vein thrombosis, related to the length of the procedure. In another pilot study in 15 patients with ET (22), unilateral thalamotomy improved tremor by 85% as a mean in the contralateral hand at 1 year; patients reported significant improvements in tremor-related quality of live from baseline to 12 months. Adverse events included only paresthesias in three patients.
Although still experimental, this technique has potential advantages over alternative techniques, since it is done without a skin incision or opening the skull. Well-designed trials with a high number of patients are still needed.
PRIMARY ORTHOSTATIC TREMOR
Orthostatic tremor is a disabling movement disorder manifested by postural and gait disturbance. The condition affects mainly elderly people. The primary treatments are medications that are often ineffective, and it can be progressive in some patients. There are only few reports in the literature describing the effects of VIM-DBS in primary orthostatic tremor. A 75-year-old patient experienced an 80% subjective improvement of tremor in the left leg and 50% improvement in the right leg. The patient was able to stand in place for several minutes before needing to sit and no longer required his portable stool/cane. An EMG after surgery showed immediate onset of tremor in the lower extremities with less continuous, less rhythmic, and of a slower frequency tremor bursts (23). Similar good results obtained have been reported at the long term in another single patient (24); there is a report of a patient improving with spinal cord stimulation at the cost of permanent paresthesias (25).
TREMOR IN MULTIPLE SCLEROSIS
Drug resistant tremor is an important source of disability in patients with multiple sclerosis (MS). There are only a few studies with a low number of patients, and short-term follow-up assessing the role for DBS to treat this problem (26–33). Functional improvement is, in general, related to the severity of tremor and coexistence of other neurologic symptoms. Five patients with MS-associated tremor were implanted with unilateral electrodes at the VIM; tremor suppression was obtained in three of five patients. New brain stem plaque formation was seen several weeks after surgery in one patient who had an acute worsening of MS which improved after high-dose intravenous steroids. Improvement of postural and action tremor was reported in 14 MS patients after thalamic stimulation, but 1 patient experienced an exacerbation of her MS 3 days after surgery, and a second patient developed an intracerebral thalamic hematoma. The follow-up was less than 1 year in all cases except one. A study showed MS improved of tremor, but speech impairment in three patients implanted bilaterally. In a prospective study in 10 patients, there was a significant reduction in the severity of tremor and improvement in hand function 12 months after the implantation of unilateral electrodes. The authors suggested that these patients were implanted at the subthalamic area/zona incerta, which would interrupt the dentato-VIM projections. Indices of disability, handicap, neuropsychological function, and independence were not improved. Two patients had thalamo–capsular hemorrhages at the site of electrode implantation, and two had seizures in the follow-up period. A prospective study showed that thalamic DBS significantly improved tremor in and ability to feed themselves in 12 MS patients but without changes in the general quality-of-life measures. The authors suggested that younger patients with MS tremor who had shorter disease duration and no superimposed ataxia benefited most from this surgery. In a study with 10 patients suffering advanced MS-related medication-resistant tremor with unilateral VIM-DBS (bilateral in one), it was observed a reduction of tremor greater than 50% at 1 year in half of the patients, but after 3 years, only 3 of them continued benefiting from stimulation. This level of improvement could be related to the variability of the demyelinating lesions and the superimposition of ataxia in these patients. It has been also suggested the need of reprogramming to maintain effects over time, due to underlying disease progression.
It was observed that MS and tremor could be better treated by simultaneous stimulation being one electrode at the VIM and ventralis oralis posterior (VOP) nuclei border and the other at the ventralis oralis anterior (VOA)/VOP border (32). Other targets, such as the zona incerta, have also been used for the treatment of action tremor in MS with good clinical results (34).
In summary, the efficacy of thalamic DBS in MS-associated tremor is variable and the impact in quality of life seems to be poor (35). It is probable that the minor benefits of DBS on disability reflect persisting cerebellar dysmetria. Besides, there can be additional difficulties with the identification of the optimal target during operation and significant procedural morbidity. Given the complexity of the underlying illness, patients should be selected carefully among those with only slight clinical repercussion of the underlying condition. Given the progressive nature of MS, long-term evaluation of the effects of DBS on tremor are crucial.
THALAMIC, HOLMES (RUBRAL), AND POSTTRAUMATIC TREMORS
Holmes tremor is an irregular, low-frequency rest and intention tremor, enhanced by posture. It can be due to vascular, traumatic, or tumor lesions disturbing the dopaminergic and cerebellar outflow systems; thalamic tremor is a mixture of intentional tremor and dystonia, following lateral posterior thalamic stroke; posttraumatic tremor usually involves the upper extremities and often exhibits a combination of rest, postural, and kinetic components. There is a delay between the head trauma and the appearance of tremor and is usually associated to other movement disorders such as dystonia or myoclonus. Most likely, it is due to lesions involving thalamus, midbrain, red nucleus, cerebral peduncle, or cerebellum.
There are several reports on single cases or small series of patients showing a good effect on tremor attenuation after VIM-DBS, in general, after a short follow-up, in patients with predominantly kinetic unilateral tremor (defined as thalamic or rubral) due to focal contralateral vascular lesions (36–39). A report on three patients showed long-term benefit of thalamic stimulation. In two of them a high pulse width was used for stimulation; curiously one of the patients continued to show tremor improvement after the end of life of the internal pulse-generator battery. In all cases, the effect on positional tremor seems to be greater than that seen on the kinetic component (40).
There are also several cases reported in the literature showing the effects of DBS on posttraumatic tremor. The most frequent target is the VIM nucleus, but dual stimulation of the VIM and the VOP/VOA (41) and the zona incerta and the VOP/VOA (42) have also been applied; stimulation of the zona incerta/posterior subthalamic region (43) has also been reported. Overall, the efficacy of this technique including all the different targets fluctuates from 38% to 67% reduction of tremor. In a recent review, nine posttraumatic tremor patients were reported to respond satisfactorily to VIM-DBS (six unilateral, three bilateral) (28).
FRAGILE X–ASSOCIATED TREMOR/ATAXIA SYNDROME
This is an inherited, X-linked, adult-onset neurodegenerative disorder caused by an expanded trinucleotide repeat in a noncoding region of the fragile X mental retardation 1 (FMR1) gene. The clinical manifestations of fragile X–associated tremor/ataxia syndrome (FXTAS), which occur predominantly in men, include action tremor, gait, and limb ataxia, probably due to dysfunction of the dentate–rubro–cerebellar loop, but also cognitive and neuropsychiatric dysfunction, parkinsonism, dysautonomia, and peripheral neuropathy. Action tremor can be invalidating and resistant to drug therapy. In recent times, a few cases have been published showing satisfactory results after thalamic DBS; however, ataxia and other symptoms associated to the disease are progressive (44–46).
TREMOR ASSOCIATED WITH NEUROPATHIES
A 30% improvement after VIM-DBS was also reported in a patient suffering a severe demyelinating neuropathy (47); remarkable clinical improvement has been shown in patients with tremor and severe neuropathy related to anti-myelin-associated glycoprotein (MAG) antibodies (48,49) and monoclonal gammopathies (50,51).
GILLES DE LA TOURETTE SYNDROME
Patients with Gilles de la Tourette syndrome (GTS) usually respond to medical treatment, and the condition often improves during adolescence; however, surgery has been considered a possible approach for the subset of patients with ongoing medically refractory disease. In 1999, thalamic DBS was introduced for intractable GTS. Multiple anatomical targets have been proposed, such as the globus pallidus internus (GPi) in either the posteroventrolateral (somatosensory) region or the anteromedial (limbic) region, the internal capsule/nucleus accumbens and the midline thalamic nuclei, with electrodes positioned at various points along the anterior–posterior axis (centromedian [CM] nucleus, parafascicular [Pf] nucleus, and nucleus ventrooralis internus). Some patients have received concomitant stimulation of both the thalamus and the pallidum, and a few patients had unilateral procedures.
THALAMIC CM/PF COMPLEX
After the first description of three patients with GTS receiving successful thalamic DBS (52), several groups have targeted this nucleus. A prospective double-blind crossover trial of bilateral thalamic DBS was conducted in five adults with TS finding consistent improvement in motor and sonic tics and quality of life in three of them (53). Reports of single cases or small series suggested efficacy of this target in diminishing tics severity ranging 46% to 70% (54–57). A large study on 18 patients including on–off and blinded evaluations in some patients, with a variable follow-up ranging from 3 to 18 months, showed improvement of motor and phonic tics, social impairment, obsessive–compulsive symptoms, self-injurious behavior, premonitory sensations, and anxiety. All patients responded well to DBS, although to differing degrees (58). Persistent amelioration of tic severity and neuropsychiatric symptoms has been reported at long-term follow-up by the same group (59,60). The long-term effects of thalamic DBS were also assessed in two patients who presented sustained improvement of tics after 10 and 6 years, suggesting that thalamic stimulation might provide sustained benefits (61). The same group tested the stimulation of the CM-Pf/substantia periventricularis/VOA in the thalamus with modest clinical benefit and at the cost of important side effects at 1-year follow-up (62). A study analyzed the effects of bilateral stimulation of the CM-Pf and concomitant GPi-DBS. Thalamic stimulation produced a reduction in tic severity ranging from 64% to 30%. The association of thalamic and pallidal stimulation showed no further reduction in tic severity, but pallidal stimulation alone produced better results ranging from 96% to 75% (63). By contrast, two reports on single patients found similar benefits with pallidal and thalamic stimulation (64,65). A study in eight patients operated in the thalamus, the GPi, or both found that only three of them presented at least a 50% improvement, while in two patients the electrodes were removed due to postoperative infection or lack of benefit (66). Unilateral thalamic stimulation in the VOP and VOA has also been reported to be effective for tic reduction (67).
INTERNAL PALLIDUM
The most frequently targeted area of the GPi is the anteromedial part. An open study on 11 patients showed the improvement of 10 of them after DBS. Overall, there was around 50% reduction in motor and phonic tics and six patients had a more than 50% reduction. Greater anxiety in two patients and hardware malfunction in three patients were remarkable adverse outcomes (68). An improvement in motor tic severity ranging from 65% to 96% has been reported in single cases or small series of patients (63), and remission of motor and vocal tics has been documented in single cases (69–71). Improvement of tics severity but the needing of progressive increase of stimulation intensity during follow-up has also been documented (72). Absence of benefit after DBS has been noted in a patient with tourettism and mental retardation (73).
The motor area of the GPi (posteroventrolateral) has also been proposed to treat GTS symptoms. Improvement of tics has been correlated with improved functional outcome, quality of life, and positive psychosocial changes in six patients with treatment-refractory TS at long-term follow-up (74). Less motor and vocal tics frequency has been reported in a single patient at the cost of bradykinesia of the left extremities as a permanent side effect (75). However, a comparative study found better outcomes in patients with electrodes sited in the anteromedial region of the GPi (54%) than in those with posterolateral (37%) implants (76).
NUCLEUS ACCUMBENS/ANTERIOR LIMB OF THE INTERNAL CAPSULE
Good outcomes are reported in a large series of 36 patients with GTS and DBS (60) in the 10 patients who were operated at the nucleus accumbens, being selected this target in 6 out of the 10 procedures on the basis of a persistent behavioral comorbidity in spite of a good response over tic frequency and severity after a first DBS procedure in the thalamus or the pallidum. Similarly, it is reported around 50% improvement in motor tics, coprolalia, and self-injuries in a patient with remarkable psychiatric comorbidity receiving nucleus accumbens DBS (77) and marked improvements in several other single cases (78,79). A 90% improvement in obsessive symptoms and a 57% improvement in tic severity have been seen in a similar case (80). By contrast, worsening in specific scales was observed in a single patient with mild vocal and motor tics in a patient with GTS and severe comorbid obsessions (81). A patient previously operated in the central thalamus and posteriorly implanted in the internal capsule/accumbens did not show substantial benefit (82).
OTHER TARGETS
Stimulation of the external pallidum (83) and the subthalamic nucleus (STN) (84) has shown improvements in tics severity in anecdotal reports that have not been replicated so far.
Given the complexity of the symptoms associated to the disease, the different brain areas that have been targeted, the relative small number of patients treated so far, and the heterogeneity of the clinical results, it becomes evident the needing of multicentric clinical trials to definitively assess the efficacy of DBS in the treatment of pharmacologically refractory GTS.
DYSTONIA
Selective peripheral denervation by repeated injections of botulinum toxin is the current mainstay in the therapy of dystonia. If this approach fails, because too many or difficult-to-reach muscles are involved, the dystonic pattern is too complex or neutralizing antibodies evolve, the treatment becomes notoriously difficult. Antidystonic drugs are typically of limited benefit. Because dystonia is thought to arise from abnormal neuronal activity in central motor circuits, neurosurgical interventions have been applied in severe cases of generalized or segmental dystonia as a last resort since the early days of stereotactic and functional neurosurgery. In the past, thalamic nuclei or the pallidum was used as lesional targets in various types of dystonia. The target for thalamotomy in treating dystonia has been much more variable among different neurosurgeons than the thalamic target for tremor. Thalamotomy has involved the VOP, the VOA, the nucleus ventrooralis internus, the VIM, the subthalamic region, the CM/Pf, and the pulvinar thalami. Several publications from the 1960s to 1980s document impressive results of stereotactic surgery in individual cases, but the therapy remained largely experimental and was restricted to few expert centers in the United States, Japan, and Europe. There were no clinical studies that would meet today’s standards: Single cases or clinical cohorts were reported by the treating neurosurgeon without independent confirmation of outcomes and adverse effects, efficacy was not quantified on scales, dystonia of different etiology were combined in clinical series, and long-term results were scarce. Therefore, the reported efficacy and safety profile of pallidal or thalamic lesioning need to be interpreted with great caution from today’s perspective. Retrospective long-term results of thalamotomy suggest a good outcome in 25% of patients and moderate success in 45%. However, 20% of the patients suffered from severe complications of surgery. Dysarthria was common, in particular in bilateral procedures, which could amount to akinetic mutism in the worst cases.
In the 1990s, pallidotomy celebrated a renaissance for the treatment of Parkinson’s disease. There was observed a profound impact of pallidotomy on contralateral off-period dystonia and levodopa-induced dyskinesia that triggered de novo interest in this target for the treatment of dystonia. In parallel, DBS evolved as an alternative to lesional stereotaxy. A milestone in the treatment of dystonia was the description of a young girl successfully treated by bilateral high-frequency stimulation of the GPi in 1996 (85). The beneficial effect of pallidal neurostimulation was soon confirmed by several surgical movement disorder groups. Since these days, target and principles of stimulation have not significantly changed and an estimated several thousand dystonia patients have been treated by DBS worldwide. Other surgical options for dystonia such as peripheral denervation surgery or intrathecal baclofen have very limited indication in cases not suitable for DBS surgery, which is now the preferred interventional therapy for various dystonia types. The degree of symptom reduction by GPi stimulation and the level of evidence-based recommendation differ among the various types of dystonia as discussed in the subsequent paragraphs.
PRIMARY GENERALIZED OR SEGMENTAL DYSTONIA
Two randomized controlled clinical trials provide level I evidence in favor of pallidal stimulation for the treatment of primary generalized and severe segmental dystonia. The French SPIDY study assessed motor improvement, quality of life, and mood after GPi stimulation in 22 patients with primary generalized dystonia (86). Average motor improvement on the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) was 47% after 3 months, 51% after 6 months, and 55% after 12 months. Involuntary facial movements and dystonic speech problems did not change significantly. At 3 months, stimulation was temporarily switched off for 10 hours, which significantly worsened dystonic symptoms, but they remained less severe than before surgery. This finding contrasts with the rapid reversal of neurostimulation effects in tremor or Parkinson’s disease and additional plastic adaptive changes in dystonia. The disability scale showed an improvement of 34% at 3 months, 42% at 6 months, and 44% at 12 months, respectively. The quality-of-life SF36 score was significantly improved after 12 months in the subscores general health and physical functioning. Patients were screened for neuropsychological and psychiatric safety, and appropriate scales did not reveal any change in these domains. In a subgroup analysis, motor outcome did not differ between DYT1-positive and negative patients. A follow-up of the original study population found motor improvement and quality of life to be preserved for up to 3 years (87).
The German multicenter study on DBS in primary generalized and severe segmental dystonia included video-based blinded assessments and a placebo control (88). Forty patients were implanted with a neurostimulation system and were randomized to either treatment or sham treatment after surgery for a period of 3 months. The change from baseline on the BFMDRS was significantly greater in the neurostimulation group (−15.8 ± 14.1 points) than in the sham-stimulation group (−1.4 ± 3.8 points, p < 0.001). After the 3-month evaluation, all patients continued on effective neurostimulation. Six months of neurostimulation led to a 48% reduction of motor symptoms compared to baseline. In this study, phasic movements responded often within hours to GPi-DBS, while tonic postures required weeks or months to improve. Remarkably, there was a great variability in motor outcome among patients from less than 25% to more than 75%, with most patients ranging from 25% to 50%. No predictive factor for favorable outcome could be identified. There was neither a difference between segmental or generalized dystonia in proportional motor benefit nor between DYT1-positive or negative patients. Again, the procedure was found to be cognitively safe and led to a reduction of preoperative depressive symptoms. Thirty-two of the patients in the original trial could be followed for up to 5 years (89). Motor improvement (BFMDRS) was 61% at 3 years of stimulation and 58% at 5 years compared to baseline. The improvement from 6 months to 3 years was significant. These long-term data suggest continuing improvement after surgery for up to 3 years and sustained benefit thereafter. A progressive improvement was limited to the patients with generalized dystonia and may relate to the greater prevalence of fixed dystonic deformities in this group. Nonmotor scores remained basically stable throughout the 5-year interval. The therapy was overall well tolerated. The most frequent stimulation-related adverse event was dysarthria followed by worsening of dystonia, which could be managed by reprogramming of the stimulator. Device-related problems such as lead dislodgement, breakage, and wiring problems were more common than in tremor or Parkinson patients, which may be related to the physical stress upon the implanted material caused by the extreme body movements in dystonia.
The findings to the two randomized–controlled trials in severe primary dystonia are corroborated by two cohort studies using masked-outcome assessment (90,91) demonstrating 50% to 60% motor improvement after 12 to 36 months and various uncontrolled, open-label cohort studies.
In summary, there is ample evidence for the efficacy and relative safety of pallidal DBS in primary generalized and severe segmental dystonia. Because pharmacotherapy is usually ineffective in these cases, the surgical option should be discussed early with affected individuals. Surgery should not be delayed within the course of disease to minimize the impact of dystonia on social life (e.g., professional activities or school education) and to avoid secondary orthopedic complications.
PRIMARY FOCAL DYSTONIA
Adult-onset focal dystonia is the most prevalent forms of primary dystonia. In contrast to more widespread forms of dystonia, focal dystonia can normally be managed well by chemodenervation to correct the abnormal postures. Therefore, most clinical reports of pallidal neurostimulation in this group have focused on patients who failed on botulinum toxin treatment.
Successful pallidal neurostimulation for severe and drug refractory cervical dystonia was first reported in 1999 (92). A Canadian multicenter study studied the impact of pallidal neurostimulation in 10 patients with cervical dystonia followed for 12 months using blinded video ratings (93). Motor improvement, as assessed by the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), was 28% at 6 months and 43% at 12 months. Pain and disability scores were also improved by 66% and 64%, as well as mood (BDI) and quality of life (SF-36) by 58% and 24%, respectively. In another prospective single-center study, eight patients with cervical dystonia were followed for up to 48 months (mean: 30 months, range: 12–48 months) (94). This study reports a median reduction in the TWSTRS motor score of 73% at the last follow-up, which was further improved compared to 50% at 6 months. In the TWSTRS nonmotor symptoms scores “disability” and “pain,” a median improvement of more than 90% was reported at last follow-up as well as significant improvements in SF-36.
A number of case reports and small series confirm the principle efficacy of pallidal neurostimulation for dystonia in other body regions, including truncal dystonia (95–97) and Meige syndrome (98). Of concern are reports about parkinsonian symptoms, such as micrographia, bradykinesia, or gait freezing induced by GPi stimulation. These adverse effects are often mild and well tolerated in return for the marked benefit of DBS, but may become clinically relevant in some patients (99,100). It is still enigmatic if these adverse effects relate to particular electrode positions within the GPi or patient intrinsic factors. Several groups have suggested using the STN as an alternative target for dystonia to avoid stimulation-induced bradykinesia (101), and a small randomized comparison did indeed favor the STN over the GPi (102). The results, however, are still preliminary, and transient dyskinesias are a frequent adverse effect of STN-DBS in dystonia (101), which need to be balanced against the pallidal risk of bradykinetic symptoms.
Despite promising result, DBS of the GPi or STN is still considered last resort treatment for patients who have developed neutralizing antibodies against botulinum toxin or in whom the movement pattern is too complex to be managed well by chemodenervation of individual neck muscles. There has been no head-to-head comparison of surgery and botulinum toxin treatment. The low, but potentially serious risk of surgery, however, will preclude using DBS as a first-line treatment.
DYSTONIA PLUS
The term “dystonia plus” comprises different hereditary disorders with a combined phenotype of pure dystonia and myoclonus or parkinsonism.
Myoclonus dystonia (MD) due to ε-sarcoglycan mutations is of autosomal dominant inheritance with reduced penetrance related to maternal imprinting. Only the myoclonic jerks but not the dystonic postures (mostly segmental dystonia affecting neck and arm) respond to alcohol. In a homogeneous group of five patients, GPi stimulation improved myoclonus by 87% and dystonia of 85% after 6 to 9 months, which remained stable up to 18 months. No adverse events were documented (103). It has been evaluated the effect of combined thalamic and pallidal neurostimulation in 10 patients with MD had found no or little additional benefit of thalamic stimulation for up to 62 months after surgery (103,104).
Several case reports but no formal study describes a beneficial effect of pallidal or combined pallidal–subthalamic stimulation in dystonia–parkinsonism (105,106).
SECONDARY DYSTONIA
Secondary dystonia are a very heterogeneous group of disorders, in which dystonia results from an exogenous cause, including perinatal hypoxic injury (cerebral palsy), head injury, or heredodegenerative disorders (e.g., neurodegeneration with brain iron accumulation [NBIA] or Wilson’s disease). Only few studies have evaluated the effect of GPi-DBS on particular types of secondary dystonia. A retrospective study collected retrospective outcomes in 23 patients with NBIA (60.9% with PKAN-mutation). The severity of dystonia was improved by 28.5% at 2 to 6 months after surgery and 25.7% at 9 to 15 months (107). Half of the patients improved by 20% for dystonia, and one-third of them improved for disability. Quality of life was improved by 83%.
The movement disorders caused by cerebral palsy are often complex and may include spasticity, ataxia, or hypotonia in addition to dystonia. Cognition can be within normal range. A prospective multicenter study (108) assessed effects of GPi-DBS in 13 adults with CP. This treatment group had mainly dystonia and choreoathetosis, but little spasticity or ataxia, normal cognitive function, and only minor damage to the basal ganglia as assessed by MRI. In this study, the mean reduction of dystonia severity as measured by the BFMDRS was 24%. Even in this clinically much preselected group of patients, treatment effects were heterogeneous, reaching from −7.5% (worsening) to 55% (improvement) on the BFMDRS after 1 year of GPi-DBS. The full impact of DBS in cerebral palsy may only be visible in children, because earlier symptom control may improve motor development and thus avoid some permanent disability. A randomized–controlled multicenter study is currently under way in Germany.
ALTERNATIVE SURGICAL PROCEDURES
Selective peripheral denervation surgery (Bertrand’s procedure) has been advocated for patients no longer responding to botulinum toxin injections due to neutralizing antibodies. Beneficial results have been reported in the range of 70% to 90% of patients in most series, with only few persistent side effects (109,110). A prospective study, however, found less favorable long-term results with frequent dysphagia and demonstrated reinervation after initially successful selective peripheral denervation (111). A recent comparative study of DBS and selective peripheral denervation demonstrated more pronounced benefit with neurostimulation, which was also effective after previous failed peripheral denervation surgery (112). Selective peripheral denervation surgery may still be considered as an alternative for patients with excellent previous response to botulinum toxin treatment and a limited number of muscles involved in the abnormal head postures or for those patients not willing to undergo brain surgery. The surgery, however, requires special neurosurgical training and will remain restricted to few expert centers.
Intrathecal baclofen has been used for almost two decades in patients with generalized dystonia (113). Patients may be screened with bolus injections of baclofen via lumbar puncture or by continuous infusion via an external micropump and an intrathecal catheter. When the dystonia responds to the testing, a programmable subcutaneous pump is implanted and connected to an intrathecal or intraventricular catheter. With the advent of DBS, the procedure has been mostly applied in patients with severe secondary dystonia and additional spasticity. Despite promising reports in individual cases, long-term results and independent confirmation in controlled clinical studies are still lacking.
PATIENT SELECTION FOR DBS IN DYSTONIA
The clinical heterogeneity of dystonia and rapidly expanding indications for DBS surgery can make it difficult for a neurologist to decide which patients could be good candidates for invasive treatment and should be sent for further evaluation to a surgical center.
An exact etiologic classification of dystonia is the most important factor for further treatment decisions, because primary dystonia respond in general more favorable to DBS than secondary ones. However, even limited motor improvement can have a major impact on quality of life in secondary dystonia, if a patient regains autonomy in certain activities of daily living, such as feeding or wheelchair control. Therefore, the decision for or against surgery requires extensive discussion with the patient and caregivers in which realistic treatment goals need to be defined and balanced against the individual risks of surgery. This includes the identification of the predominant source of disability and an evaluation of the likelihood to become improved by GPi-DBS (114).
There are only few studies attempting to define predictive factors of GPi-DBS in dystonia. In an analysis of 44 patients with primary dystonia without skeletal deformities, the only predicting factor of a better outcome was shorter disease duration (115). The time required to achieve maximal clinical response took longer in older patients, but there was no difference in the magnitude of benefit compared to younger patients. The role of the genotype is currently debated. Patients carrying the DYT1 mutation were thought to be ideal candidates for DBS, but treatment failures have also been reported in this group (89). Patients carrying the THAP1 mutation (DYT6) often suffer from prominent oromandibular dystonia and speech problems, which are in general less improved by pallidal neurostimulation. Whether DYT6 dystonia per se is less responsive to pallidal DBS remains a matter of debate. Three patients suffering from DYT6 dystonia failing on DBS surgery have been reported (116); nonetheless, other groups have found at least moderate benefit even in this patient group (117).
A careful clinical analysis of treatment failures in the German multicenter study did not reveal any consistent predictors of poor outcome. It rather suggested that a primary or secondary nonresponse to DBS is likely the result of an individual combination of multiple possible factors, which may include suboptimal lead placement, programming issues, or disease heterogeneity. Therefore, when informing patients about DBS, physicians should not only discuss the average motor improvement obtained in clinical studies. More importantly, patients should be informed about the variability of outcomes in dystonia and the risk of a treatment failure (as defined by less than 25% motor benefit), which is in the order of 25% for primary generalized or segmental dystonia in experienced centers (89).
CHOREA
A recent prospective study in patients with chorea–acanthocytosis has shown beneficial effects of GPi-DBS in motor symptom severity, chorea, and dystonic symptoms as well as in functional capacity; effects on dysarthria and swallowing were variable. Parkinsonism did not improve (118).
Apart from single case reports, a prospective open-label study in seven patients with Huntington’s disease has shown that pharmacologically resistant chorea producing functional impairment is significantly ameliorated after GPi-DBS (119). The mean improvement on the chorea subscore was 59.8% at the 3-year visit. However, bradykinesia and dystonia showed a trend toward progressive worsening probably related to both disease evolution and DBS.
Thus, GPi-DBS may provide sustained chorea improvement in selected patients before progression of behavioral and cognitive disorders.
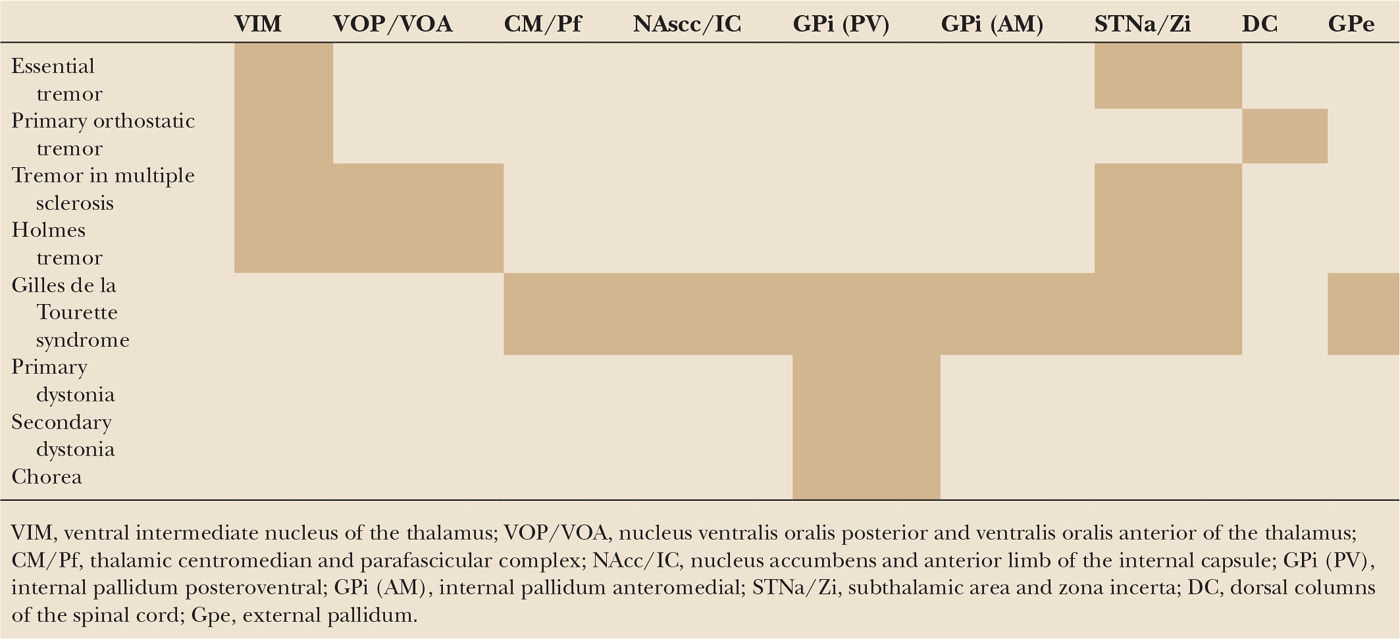
A summary of the targeted brain areas to treat hyperkinetic movement disorders is given in Table 48.1.
| Proposed Brain Targets for the Symptomatic Treatment of Different Hyperkinetic Movement Disorders |