CT
MRI: T1
MRI: T2
MRI: FLAIR
MRI: QWI
MR spectroscopy
Hypodense calcifications – rare
Hypo-/isointense
Hyperintense
Moderate hyperintense
High signal
Elevated lactate peak
No adjacent bony reaction
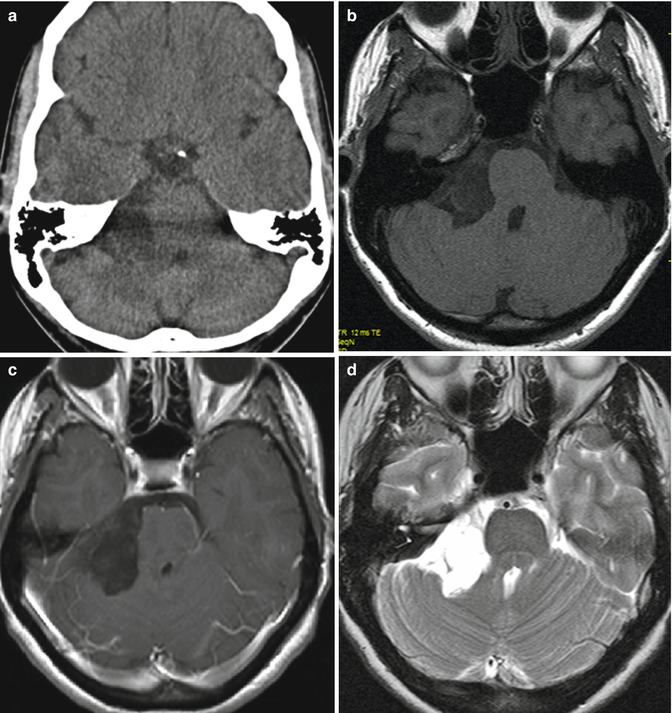
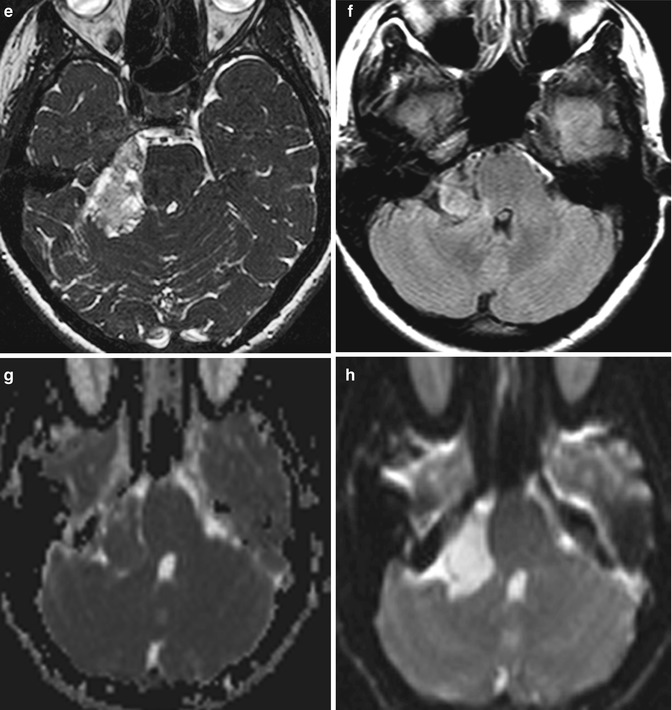
Fig. 30.1
Preoperative images of a CP angle epidermoid: (a) CT image; (b–h) MRI sequences. (b) T1-weighted sequence without and with gadolinium enhancement (c); (d) T2-weighted sequence; (e) CISS sequence, (f) T2 proton density sequence; (g) ADC; (h) diffusion sequence
30.2 Clinical Presentation
CP epidermoids are very slow-growing lesions that cause insidious and protracted course of symptoms and signs due to compression or irritation of neurovascular structures. The typical patient is around 40 years old and presents with a long-standing history of tinnitus and hearing loss. More rarely patients have headache, hemifacial spasm, trigeminal neuralgia, cerebellar and pyramidal signs, facial weakness, lower cranial nerve dysfunction, or double vision. Vestibular symptoms, however, are rare [13]. Epidermoids cause trigeminal neuralgia and facial nerve signs more frequently than other CP angle tumors [18]. Although rare, cyst rupture may cause recurrent episodes of aseptic meningitis and lead to hydrocephalus [1, 5].
30.3 Management
The only treatment option of epidermoids is surgical; surgery may be curative, provided complete removal of the lesion is achieved. The intimate relation of CP angle epidermoids to critical neurovascular structures, however, may be a limitation to safe radical surgery. Furthermore, the cyst capsule may be very densely adherent to brainstem, some cranial nerve, or important vessel. Various approaches have been proposed and utilized according to the epidermoid extension, such as the translabyrinthine, translabyrinthine-transcochlear, middle fossa, combined pre- and subtemporal-transtentorial, subtemporal-transtentorial, frontotemporal, and orbitozygomatic approaches [3, 12].
We, as well as many other authors [10, 18, 22], believe that the simple and flexible retrosigmoid approach is sufficient for the removal of CP angle epidermoid of any size, even those extending beyond the CP angle. We discourage from utilization of hearing destructive transpetrous approaches, such as the translabyrinthine or translabyrinthine-transcochlear, because hearing can improve or even recover after tumor removal.
30.4 Retrosigmoid Approach
Detailed description of the retrosigmoid approach has been provided in several earlier publications [18]. Therefore, only the key steps are going to be presented here.
Preferred patients’ position is the semi-sitting, which enables the surgeon to use both his hands for tumor removal/dissection. The necessity of constant suction or frequent coagulation during tumor removal is obviated: the irrigation fluid performed by the assistant clears the operative field and drains out spontaneously. Another important advantage of this position is the decreased engorgement of the cerebellum.
The head is fixed with a pin holder; then, it is extended, flexed, and rotated to the side of the lesion. Tumor location and extension determines the degree of rotation (approximately 30–45°). Occlusion of venous jugular outflow or hyperflexion of the cervical spine should be avoided. A drawback of the semi-sitting position is the risk of venous air embolism, paradoxic air embolism, tension pneumocephalus, or circulatory instability. In experienced hands with good coordination between the individual team members, however, these are not related to any lasting morbidity. Transesophageal echocardiography is the most specific and sensitive method for detection of air embolism. Similar outcome may be achieved even with the technically more convenient combination of end-tidal carbon dioxide monitoring and precordial Doppler echocardiography. If immediate measures are carried out at the first sign of venous air embolism, it can be well controlled and the related morbidity is insignificant.
Important prerequisite for safe surgery is the continuous neurophysiological monitoring, which should be performed throughout the surgery: from the positioning of the patient to the skin closure. Monitoring of somatosensory evoked potentials is especially important during patient positioning in order to prevent spinal cord compression. Preoperative functional X-rays of the cervical spine could determine the patients at risk, such as those with severe degenerative spine disease or spinal instability.
Monitoring of the brainstem auditory evoked potentials (BAEP) provides instant feedback information on the functional status of the cochlear nerve during tumor removal. The loss of any wave can be prevented by early recognition of its deterioration and modification of surgeon’s actions. Using direct electrical stimulation identifies the facial nerve; its functional integrity can be monitored continuously by electromyography or facial nerve motor evoked potentials monitoring. Monitoring of the oculomotor, trochlear, abducent, and/or the lower cranial nerves is performed if needed – according to the particular tumor extension and clinical presentation.
A slightly curved skin incision is performed 1.5–2 cm medial to the mastoid process. We avoid making one-piece craniotomy due to the related high risk of injury to the underlying sinuses and tearing the dura by the craniotome. The borders of the sigmoid and transverse sinuses are exposed: more extensive exposure of these sinuses is not necessary and may lead to their laceration or desiccation with the risk of subsequent thrombosis. The bone opening should extend to the floor of the posterior fossa. In caudally extending epidermoids opening of the foramen magnum may be necessary. The dura is incised in a curvilinear manner slightly medial to the sigmoid and inferior to the transverse sinus; usually this allows for a primary watertight dural closure. The initial intradural step should always be the draining of CSF by opening the lateral cerebellomedullary cistern: the cerebellar retractor should not be placed until the sufficient CSF is released.
In epidermoids – in contrast to other CP angle tumors – the cranial nerves and/or major vessels are frequently engulfed by the tumor and dislocated to a lesser extent. Importantly, the long-standing compression may alter their morphology and render them more vulnerable. Medially located epidermoids are removed consequently via the CP angle levels: between the tentorium and the 5th nerve, between the 5th and the 7–8th nerve complex, between the 7–8th and the lower cranial nerves, and between the latter nerves and foramen magnum. The tumor is removed completely in one region before moving to the next one, thereby minimizing the risk of overlooking a remnant. The cranial nerves may be identified near their bony or dural entrance and dissected in a lateral-to-medial direction.
Tumor removal is performed using simple suction (at lower suction power), tumor forceps, microdissector, and/or platelet knife. The use of ultrasonic aspiration is not recommendable because it can easily damage neural structures.
The general concept for removal of skull base tumors should be followed: initial internal decompression followed by dissection of the tumor capsule. The application of the two-hand technique under high magnification has proven very useful for the dissection of the capsule.
In regard to the goal of surgery – whether to attempt always complete removal of the capsule or not – the opinions are controversial. Some authors favor always complete removal of the capsule to prevent recurrence [2, 22], while other recommend more conservative approach to minimize operative morbidity and mortality [8, 18, 19]. Our concept is that small remnants of the capsule that are firmly attached to cranial nerves, brainstem, or vessels should not be removed in order to prevent additional neurological morbidity. Remnants have to be followed-up with regular imaging but their propensity for further growth is extremely low. Berger and Wilson [3] reported that it may take 30–40 years for recurrent symptoms to develop.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








