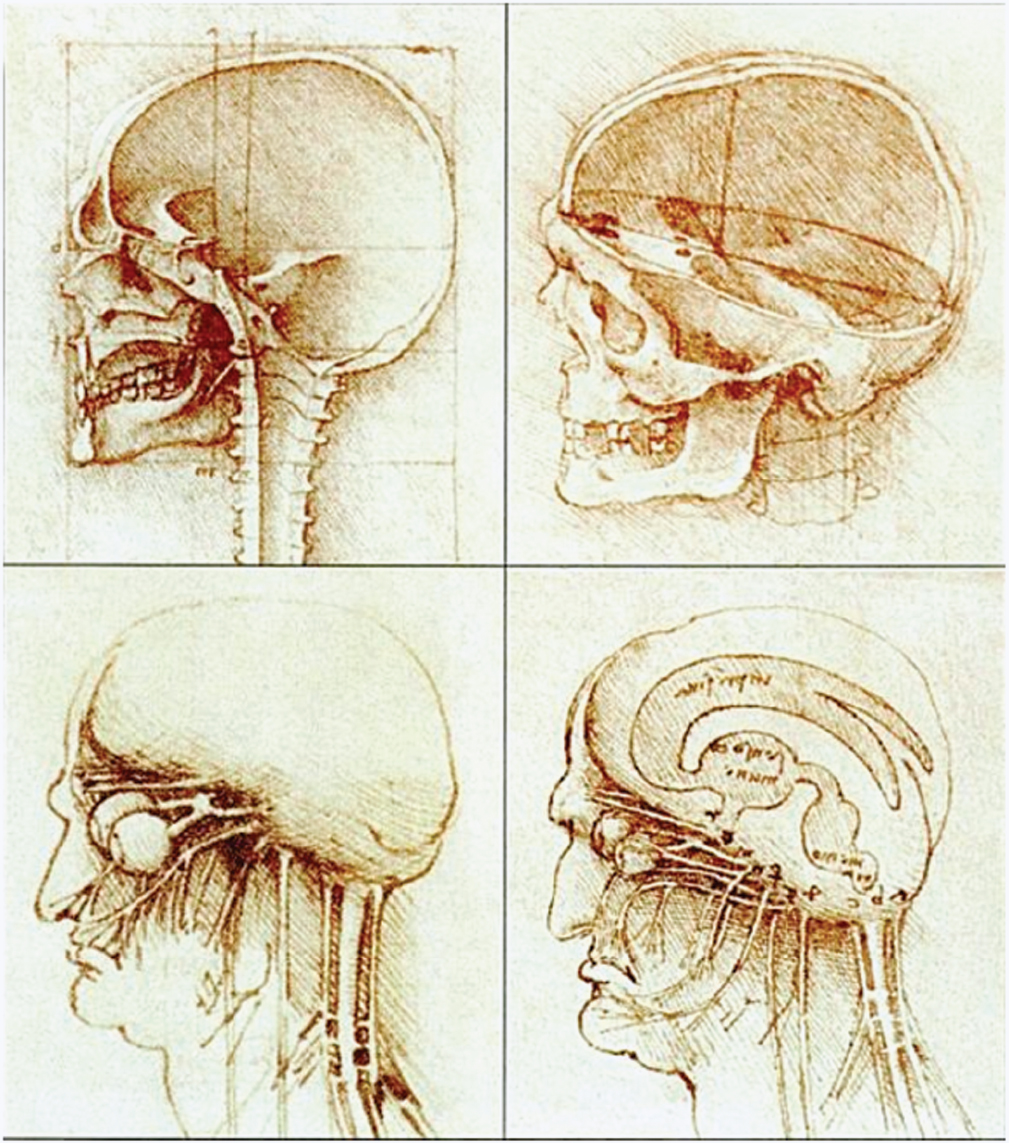
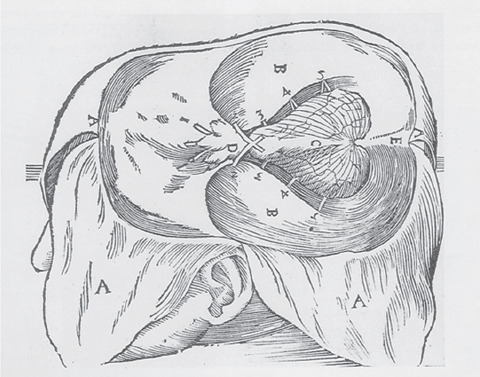
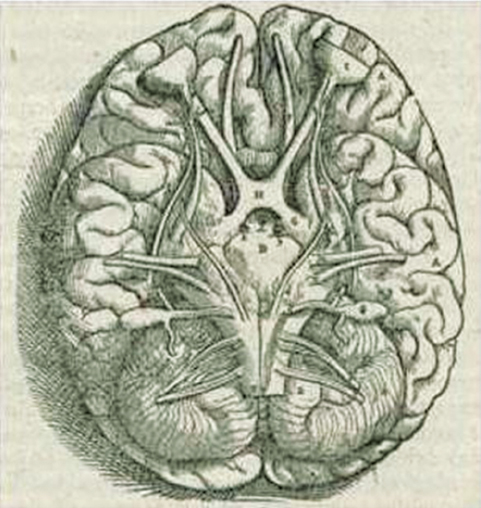
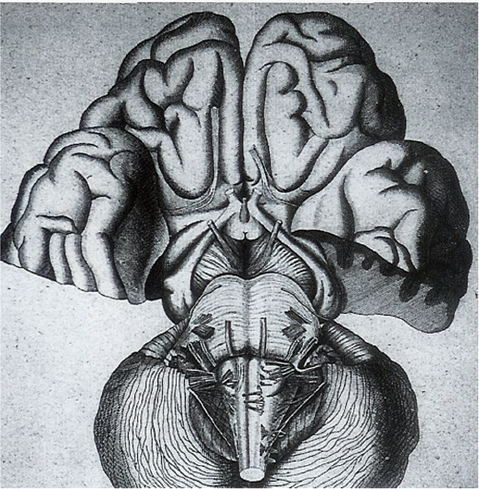
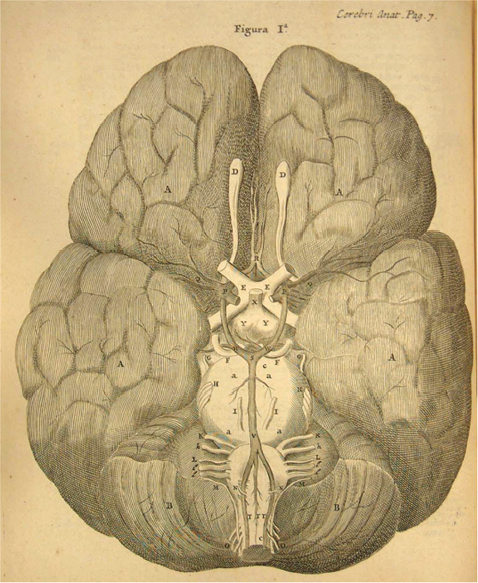
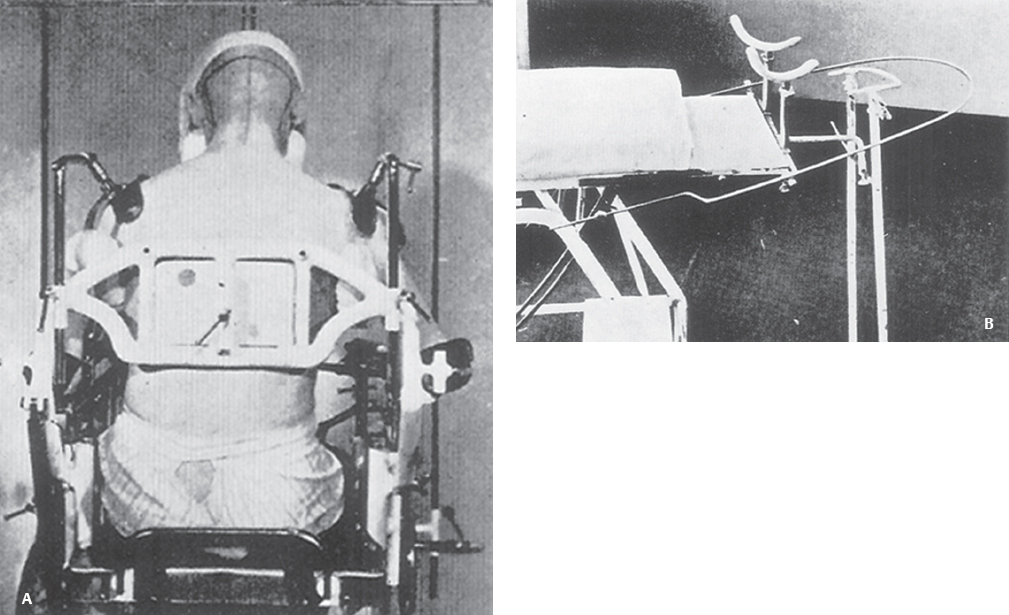
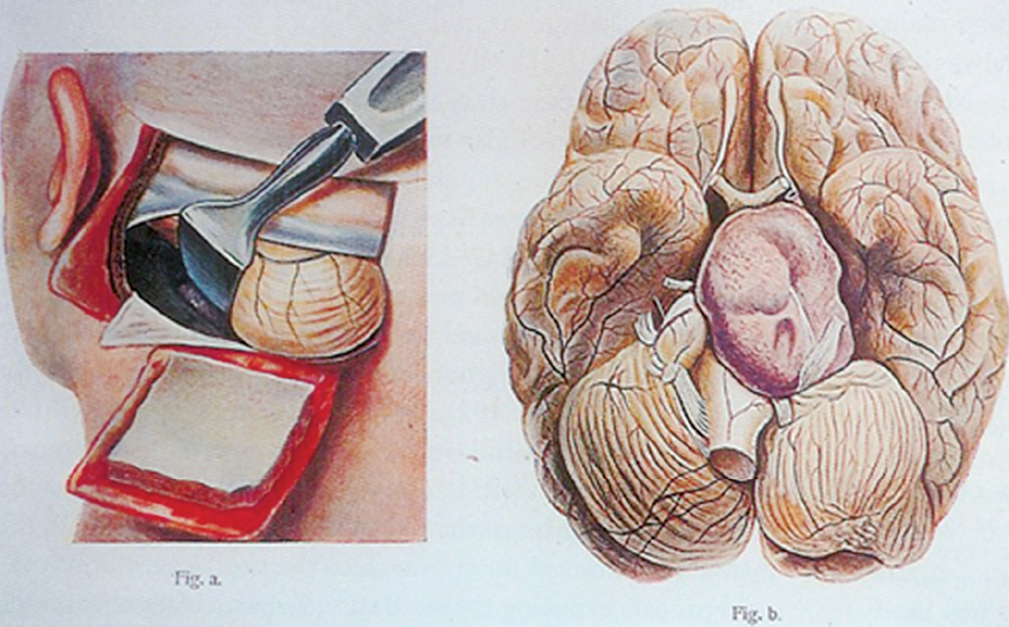
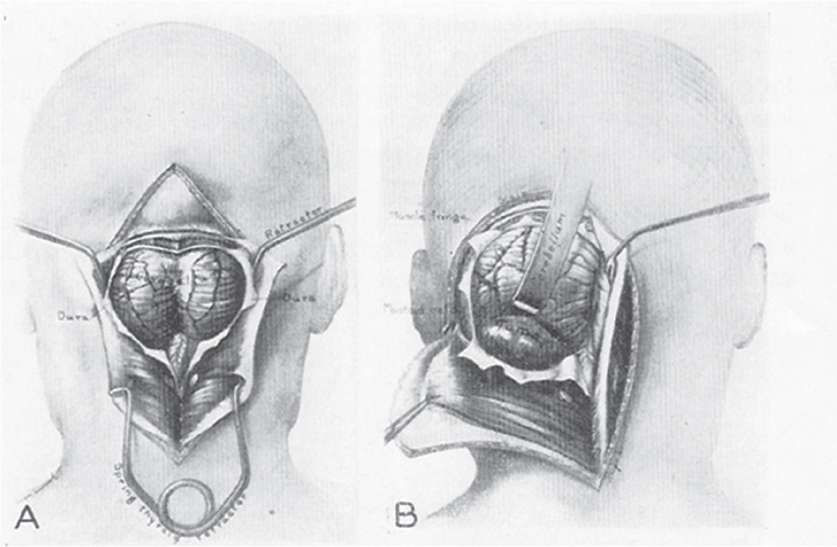
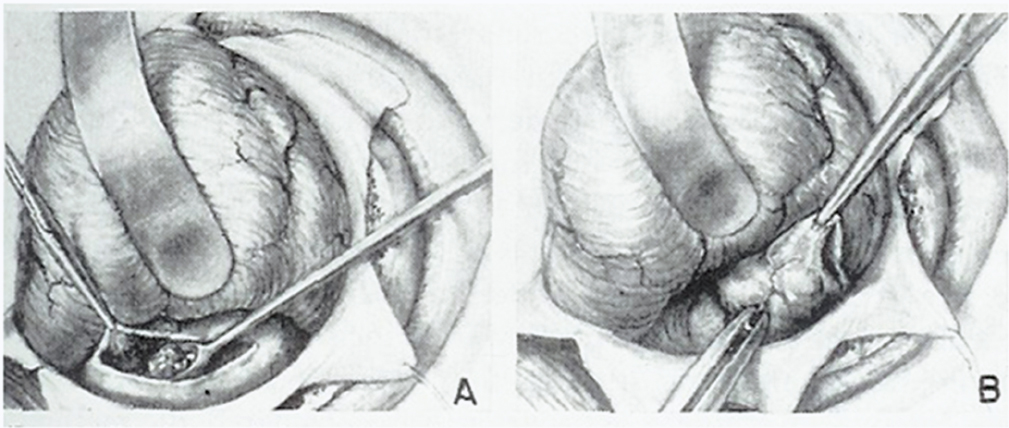
1 The earliest known surgeries performed on the cranium date as far back as prehistoric times. The vast majority of these, however, were performed on the cranial convexity. The history of posterior fossa operations, as compared with the entire history of neurosurgical procedures, is relatively brief. This is not surprising, considering the vulnerability of the vital neural structures found in the posterior fossa. Surgical manipulation of the cerebellum, brainstem, and cranial nerves resulted in forbiddingly high mortality prior to the sophisticated techniques and operative environment available in the modern neurosurgical era. Even with the diminished risk associated with contemporary technological advancements, surgical procedures involving the posterior fossa continue to carry higher morbidity than elsewhere in the central nervous system. The first successful surgeries in the posterior fossa occurred at the end of the 19th century and involved drainage of cerebellar abscesses through trephine openings behind the mastoid process.1,2 In 1893 Charles McBurney, an American surgeon best known for the eponymic landmark used in diagnosing appendicitis, reported the first successful removal of a cerebellar tumor.1,3 Surgical treatment of lesions in the posterior fossa has expanded since that time to include a wide spectrum of pathologies. The ability to successfully operate in the posterior fossa results from a detailed understanding of the neuroanatomy and physiology, accurate localization of lesions, improved surgical techniques, and the development of antisepsis and anesthesia. Today’s neurosurgeon is indebted to the numerous individuals who contributed to these elements. The first recorded technique for a posterior fossa approach can be found in the Anatomic Procedures written by Claudius Galen in AD 177.4 Galen (AD 129–200) was born in the cultural and intellectual Hellenistic center of Pergamon (Fig. 1.1). His induction into medicine has mythological origins that tell of Aesculapius, the Greek god of medicine and son of Apollo, inspiring Galen’s future career in a dream his father had. Galen went on to become the most prominent physician of his time, holding positions that included personal physician to Marcus Aurelius and his son Commodus, and surgeon to the gladiators. He made extensive contributions to the understanding of neuroanatomy, including the first recorded attempt at identifying and numbering the cranial nerves.5 Human cadaveric dissections were forbidden in the Roman Empire during the 2nd century AD, leading Galen to develop his approach to the fourth ventricle through live animal dissections.4 He chose monkeys as his primary subjects, not only for their assumed anatomic similarity to humans, but to avoid the inconvenience of having the animal “shout at the top of their lungs” during the dissection, as tended to occur with pigs and goats.4,6 Galen’s efforts were primarily for educational purposes, and there is no conclusive evidence that he ever attempted this approach in a human.4 Regardless, the methodology he developed for approaching the cerebellum and fourth ventricle is remarkably similar to the standard approach used 1700 years later by contemporary neurosurgeons. Galen recommended making a straight incision through skin and muscle in the midline of the occipital region. Blood loss was controlled by distracting and elevating the edges of the wound, and hooks were placed to keep the tissue spread apart.4,6 Following removal of the periosteum, he described a suboccipital craniectomy using special chisels to protect the dura mater. He specifically suggested to “take care not to tear the thick membrane” when removing bone, and to extend the craniectomy “until the suture shaped as the Greek letter lambda.”4,6 When explaining the dural opening, Galen emphasized elevation of the dural edges to avoid injury to the underlying cortex. The vermis was then divided to expose the fourth ventricle. Galen found in his investigations that in order for the animal to survive and remain conscious, only a small incision through the vermis could be made, as an extended division would result in irreversible impairment of consciousness.4 Fig. 1.1 Claudius Galen (AD 129–200). (From Wellcome Institute Library, London.) Much of the intracranial work done by Galen was described only in Arabic and thus was not available to the anatomists of the Renaissance.4 The precocious nature of his work is demonstrated by the superior accuracy of his descriptions of fourth ventricular anatomy to those made during the 16th century, as well as through a comparison of his surgical approach with the technique described in Charles Frazier’s 1926 publication entitled The Midline Bloodless Approach to the Posterior Fossa.7 Galen’s approach to his own education as a physician remains a relevant example for modern-day students of neurosurgery. He emphasized the importance of learning anatomy through dissections of dead animals prior to attempting surgery on living animals, and his anatomic studies were undertaken with the motivation to improve his abilities as a surgeon and physician.4 The 16th century encompassed pivotal events and key individuals whose contributions laid the foundation for modern neurosurgery. The passion for new knowledge that infused this period of time and the subsequent explosion of new discoveries marked this era as the beginning of the modern anatomic period.5 Physicians of this era began to connect disease to anatomic structure for the first time, and the desire to better understand the human body led to the reintroduction of cadaveric dissections.8 Exploration of the intricate anatomy of the posterior fossa was no exception. Leonardo da Vinci (1452–1519) is recognized as the founder of iconographic and physiologic anatomy.8,9 He conducted numerous anatomic studies on the brain and ventricular system and provided the first diagrams of the cranial nerves (Fig. 1.2). His endeavors laid the groundwork for subsequent anatomists to expand the knowledge of exact neuroanatomic relationships.10,11 The first illustration of the posterior fossa can be found among the drawings of Johannes Dryander (1500–1560), a professor of surgery in Marburg (Fig. 1.3). In 1536 Dryander published what can be considered the first textbook of neuroanatomy, containing 16 plates of his dissections of the brain.12 He was also one of the first anatomists to perform public dissections of the skull, brain, and dura mater.8,13 The most well known anatomist of the period was the surgeon Andreas Vesalius (1514–1564). Vesalius made fundamental contributions to the field of medicine through his efforts to provide the most accurate descriptions possible of the human body.14 He emphasized the importance of learning anatomy alongside a cadaver and directly applied the discoveries of his own dissections to his surgical practice.8 For his studies of the brain he noted that “heads of beheaded men are the most suitable since they can be obtained immediately after execution with the friendly help of judges and prefects.”8,15,16 Vesalius drew exceptional illustrations of the brain, which included detailed dissections of the brainstem and cranial nerves (Fig. 1.4). Two centuries later, Samuel Thomas von Soemmerring (1755–1830), a German physician and anatomist, would be the first to accurately describe all 12 cranial nerves. His drawings of the posterior fossa structures were the first to incorporate almost the same degree of detail as seen in modern-day illustrations (Fig. 1.5).8 Fig. 1.2 Leonardo da Vinci’s drawings of the skull, brain, ventricular system, and cranial nerves. His drawings provided the first diagrams of the cranial nerves. Fig. 1.3 Illustration by Johannes Dryander demonstrating the posterior fossa and cranial nerves. Fig. 1.4 Andreas Vesalius’s illustration of the brain, demonstrating the posterior fossa structures. Fig. 1.5 Soemmering’s drawings were the first to describe all 12 cranial nerves and rivaled the detail and accuracy of modern-day illustrations. The work of these and other key Renaissance figures created a basic foundation for the intense neuroanatomic investigations that followed during the subsequent period of scientific growth. Contemporary physicians began to appreciate the value that accurate understanding of anatomy held for the success of their surgical attempts. The expanded knowledge of brain anatomy, combined with more sophisticated surgical tools and generally improved medical conditions, enabled surgeons to consider attempting operations on the brain.8 Inquiry into the physiologic function of nervous structures followed their anatomic descriptions. Some of the first observations on the cerebellum were made by Thomas Willis (1621–1675). Willis, along with his study of what became known as the circle of Willis, improved the classification of the cranial nerves (he recognized nine), and he published in 1664 the most accurate text of neuroanatomy to date, Cerebri Anatomie (Fig. 1.6).5,8,17 In his text, Willis suggested that the cerebellum regulated involuntary movements.1,5 This theory was later confirmed by the Italian physician Luigi Rolando (1773–1831) in 1809 with his experiments demonstrating cerebellar control over movement. Marie Jean Pierre Flourens (1794–1867) in 1824 restricted the functions of the cerebellum to the coordination of movement with his studies involving cerebellar ablations in animals.1 In the first American textbook of neurology published in 1881, William Hammond (1828–1900) described symptoms associated with cerebellar tumors including headache, vomiting, staggering gait, and convulsions.1,18 By the beginning of the 20th century, the classic findings of cerebellar tumors (ataxia, headaches, vomiting, papilledema, and hydrocephalus) were common knowledge among contemporary neurologists, leading to more accurate and frequent diagnosis of posterior fossa tumors.1,19 The ability to correlate a functional role with a neuroanatomic structure enabled surgeons to localize lesions based on the patient’s symptomatology. Walter Dandy’s development of ventriculography in 1918 went on to supplement the physical examination for localization of posterior fossa tumors.1,20,21 Fig. 1.6 Thomas Willis’s illustration from his text on neuroanatomy. Willis recognized nine cranial nerves. Elucidation of the intricate functions of the brainstem began with the work of Julien Le Gallois (1770–1814), who in 1812 discovered the vital respiratory centers in the medulla.1 Subsequent investigators, including Thomas Lumsden and Sir Charles Sherrington, went on to describe these brainstem centers in more detail.22 Knowledge of the neurophysiologic functions of the structures of the posterior fossa was vital for the surgeon attempting a surgical approach to the area. Although the cerebellum held an important role in the fine regulation of motor activity, its function was not pertinent to life. Conversely, the vital centers contained in the lower brainstem structures had to be handled extremely delicately.1 Louis Sebastian Saucerotte (1741–1814) was one of the first surgeons to realize that certain areas of the brain were far more susceptible to injury than others. As a surgeon in the French Army, he was exposed to a myriad of head injuries, and eventually published a review that gave a detailed description of the symptoms associated with certain brain injuries. He was the first to describe loss of coordination with opisthotonos and eye rolling from a cerebellar lesion. He divided the brain into areas of susceptibility, and noted that the structures of the posterior fossa—the brainstem and cerebellum—were the regions associated with the most severe injuries.8 Insight into neurologic function rapidly expanded after investigators realized that the brain did not function as a single unit and that function could be localized. G.T. Fritsch (1838–1927), E. Hitzig (1838–1907), and Paul Broca (1824– 1880) each contributed to the concept that each part of the brain correlated with a particular function.8 Robert Bartholow (1831–1904), an American physician working in Ohio, reported in 1868 his observations in three patients with brain tumors who had clinical symptoms that correlated with his anatomic findings.8 Over the course of the 18th and 19th centuries, the concept of operating in the posterior fossa gained acceptance. Percival Pott (1714–1788), described as the first modern neurosurgeon, was a strong proponent of intervention in brain injury. He felt that infections creating compression of the brain should be removed, including in the region of the posterior fossa.8 Surgeons became increasingly comfortable with their knowledge of brain anatomy and function. Symptoms of central nervous system pathology were recognized as such, leading to more aggressive clinical management. The addition of antisepsis, anesthesia, and improved techniques for cerebral localization then set the stage for the more complicated neurosurgical approaches that would be developed during the 19th century. Walter Dandy (1886–1946) made the observation that “surgery of the brain is the outgrowth of three discoveries of the nineteenth century, namely, anesthesia, asepsis and cerebral localization.”8,23 The progress in these areas of neurosurgery led to more routine attempts to treat pathology of the posterior fossa; however, the initial associated surgical mortality was shockingly high. Herman Oppenheim (1858–1919) reported a mortality rate of 71% in patients treated for cerebellar tumors.24 Henri Duret (1849–1921) reported a slightly lower rate of 60% in 1903,19 and Charles Frazier (1870–1936) reported 42% in 1905.25 The lack of understanding of cerebrospinal fluid (CSF) dynamics along with inadequate intraoperative supportive measures such as intravenous fluids and blood transfusion likely contributed to these initial poor results.1 It is obvious that these limitations made the duration of the surgery a critical factor in patient survival, and it is not surprising that tumors were only partially resected in these early cases. Neurologic surgery did not become its own specialty until the end of the 19th century. Prior to that time the operative procedures on the brain were undertaken by general surgeons. There were many talented surgeons during this early modern era who played pivotal roles in the progress that would be made in the surgical treatment of neurologic pathology. They focused their creative efforts on developing new techniques that would push the field forward. Positioning of the patient in a manner that would facilitate suitable access to the posterior fossa presented one challenge. Victor Horsley (1857–1916) and Fedor Krause (1857–1937) recommended a lateral position on the operating table, whereas Harvey Cushing (1869–1939) and Frazier each developed their own specialized head rests to allow the patient to be placed in the prone position. Although Cushing also endorsed some elevation of the head to reduce venous pressure, French surgeon Thierry de Martel (1875–1940) introduced the sitting position and designed a special chair for use during his cerebellar surgeries (Fig. 1.7).1,8 Fig. 1.7 (A) De Martel’s operating chair, enabling a sitting position for operations of the posterior fossa. (B) Cushing’s operating table for surgery of the cerebellum, with the patient in the prone position. The incisions and muscle flaps created to approach the cerebellum varied significantly and changed a great deal before evolving into the straight linear incision that is most commonly used today. Krause, considered to be the father of German neurosurgery, was the first to publish detailed techniques for approaches to the posterior fossa. He used a unilateral or, when necessary, bilateral horseshoe flap that he reflected inferiorly (Fig. 1.8). Horsley used a curved incision from mastoid to mastoid with the apex above the inion.1,26,27 Similarly, Charles Ballance (1856–1936) used an upward-curving incision from mastoid to midline for a unilateral exposure.1,28 Cushing devised his “cross-bow” incision in 1905 in an effort to improve exposure. Starting with a curved transverse incision between the mastoids, he added a midline extension down to the lower cervical spine. Few other surgeons adopted this approach because of the additional time required to close the wound.1 In 1926 Frazier described his “midline bloodless approach to the posterior fossa.”1,7 The incisions used prior to that time caused inconvenient bleeding when cutting through the occipital arteries and veins as they crossed the midline. Frazier’s straight vertical incision avoided this problem with a simpler extension from 2 cm above the inion to the upper cervical region. He made a small (2 cm) transverse extension of the incision at the superior apex to facilitate retraction and provide a site for trephine opening and ventricular puncture should it be necessary. Howard Naffziger described an approach to expose the anterior cerebellum in 1928 involving opening the tentorium to the incisura tentorii while retracting the occipital lobe.29 Naffziger also used Frazier’s midline incision to approach midline cerebellar lesions (although he eliminated the small transverse extensions at the inion) and “hockey-stick” incisions extending from the tip of the mastoid to the inion and then vertically down the midline when approaching one cerebellar hemisphere (Fig. 1.9). Alfred Adson (1887–1951) described in 1941 the use of a straight vertical incision over the lateral suboccipital regions for unilateral cerebellar exposure, but the upward-curving mastoid-to-mastoid lateral incision seems to be the most generally popular incision used by surgeons of this era. Dandy favored this incision in a large series of cerebellar surgeries he described in 1932, and Paul Bucy (1904–1992), William Cone (1897–1959), and Wilder Penfield (1891–1976) also used this incision routinely.1 Given the thickness of muscle in the occipital area, most surgeons were not hesitant to perform suboccipital craniectomies, as there would be no deforming defect. Although many replaced the bone just as they would at the cerebral convexity, they realized early on that discarding the bone flap had the additional advantage of decompressing the posterior fossa. Fig. 1.8 Krause’s approach to the cerebellopontine angle using a hinged horseshoe osteoplastic flap. Fig. 1.9 Illustrations demonstrating the midline (A) and hemispheric (B) approaches to the posterior fossa used by Naffziger. Techniques involving drainage of CSF evolved over the course of the 20th century. The common occurrence of hydrocephalus in the setting of posterior fossa tumors was well recognized, and Krause recommended ventricular puncture to reduce intracranial pressure prior to operating on a cerebellar tumor as early as 1909.27 This practice was followed in different variations by other surgeons. Dandy routinely tapped the lateral ventricle through an occipital trephine prior to opening the posterior fossa dura to prevent cerebellar herniation.23 Several surgeons including Frazier favored lumbar puncture to reduce intracranial pressure.25 The imaging modalities available to present-day neurosurgeons allow for selecting out the patients who benefit from CSF diversion prior to or during a posterior fossa surgery. As experience with these operations grew, important operative techniques were added to the surgeons skill set, making these surgeries progressively safer. Aggressiveness with removal of a tumor in the cerebellum was tailored to the type of pathology and known benefits of complete resection, brainstem structures were treated gently, and disturbance of the deep cerebellar nuclei was avoided despite realizing that a zone of normal cerebellar tissue surrounding a tumor could be tolerated without any gross neurologic deficit. An intraoperative ventricular dye test was used by Dandy to diagnose an obstructive lesion or check for patency of the normal CSF pathways.20 A meticulous, layered closure of all posterior fossa surgeries was championed by Cushing to avoid CSF leaks and infections. All of these contributed to a progressive decline in surgical mortality and morbidity. In 1932 Cushing reported an 11.2% operative mortality rate in a series of 91 cerebellar astrocytomas and 25.3% operative mortality in a series of 68 medulloblastomas.30 In today’s operating room, surgical mortality in cerebellar tumor surgery is uncommon. The Scottish surgeon Sir Charles Bell (1774–1842) described one of the first cases of a cerebellopontine angle (CPA) tumor in 1833 after diagnosing the lesion at autopsy.8 The first attempts to resect tumors in this area began at the end of the 19th century when surgeons attempted to remove vestibular schwannomas and meningiomas. The first report of a successful operation on a tumor of the CPA is credited to Ballance in 1894 after he removed an acoustic neuroma in a 49-year-old woman who had presented with headache, vertigo, and tinnitus.8,28 Unsurprisingly, the initial attempts to approach the CPA met more commonly with failure than success. Moritz Borchardt in 1906 reported 18 cases with 13 deaths. Several years later in 1913 Anton von Eiselsberg reported 16 cases with 12 deaths, and Krause reported 31 cases with 26 deaths.1 In 1917, Cushing reported a much lower operative mortality of 15% with CPA tumors, likely due to his meticulous surgical methods.31 Many of the approaches used to access the CPA were quickly found to be unsuccessful. One example is the translabyrinthine approach suggested by R. Panse in 1904.32 Despite the successful use of this approach today, difficulty in removing larger tumors without traumatizing the surrounding structures along with the frequency of postoperative CSF leakage quickly made this approach unpopular. Charles Elsberg, Borchardt, and von Eiselsberg all attempted a combined suboccipital and petrosal approach without success. Frazier’s approach of choice involved a unilateral suboccipital craniectomy combined with an occipital bone flap that extended to the lateral sinus.1,33 Cushing not only was a talented surgeon with meticulous technique but also was responsible for many of the most important contributions to his field. Cushing realized that none of the approaches attempted by his predecessors had provided sufficient working space. This combined with the common technique of finger enucleation led to mechanical trauma of the posterior fossa structures. His approach utilized the crossbow incision, bilateral craniectomies, and drainage of CSF via tapping of the lateral ventricle. He opened the dura in a stellate fashion and retracted the cerebellum medially to expose the CPA tumor. His attempts to lower the mortality rate led him to forgo a complete resection, instead debulking the tumor through a capsular incision and leaving the capsule behind. For the larger acoustic tumors he resected a portion of the cerebellar hemisphere to obtain adequate exposure, a technique he referred to as “cerebellar uncapping.”1,31 Dandy went on to improve on Cushing’s technique, eventually favoring a smaller unilateral flap and craniectomy in the lateral position.24 Dandy trained under Cushing and made some of the most innovative and influential contributions seen in neurosurgery. The methods he developed in his approach included enucleation of the tumor from within the capsule followed by complete removal of the capsule by carefully dissecting it away from its attachments. He reported five such operations in 1934, with all five patients surviving1,34 (Fig. 1.10). Cushing and Dandy notoriously clashed over their differing approaches to treating acoustic neuromas. When Dandy neglected to mention Cushing’s work in an article describing his own technique for the Johns Hopkins Hospital Bulletin, Cushing was incensed, and wrote to Dandy, “You must not forget your manners, and this last note of yours is in extremely bad taste.”8,24 The different approaches to these tumors created some controversy in the field as surgeons attempted to weigh the consequences of facial paralysis, tumor recurrence, and surgical mortality to determine the best method for operative treatment. In general, facial paralysis was felt to be preferable to tumor recurrence, as reoperations carried even greater mortality risk. Surgeons did not begin attempting to spare the facial nerve during complete resections until Hugh Cairns reported such a success in 1931.35 With the refinement of microsurgical techniques and addition of facial nerve monitoring, the preservation of facial nerve function today has dramatically improved. Considerable progress has been made in the surgical management of posterior fossa lesions. The advances in anesthesia, antisepsis, and critical care have had a major impact on operative mortality. Introducing the operating microscope to the field of neurosurgery in the 1950s enabled revolutionary advances in technique to be made, especially when operating on the small and sensitive structures found in the posterior fossa. Stereotactic radiosurgery has demonstrated excellent results for many pathologies, and has become an important adjunct for the treatment of posterior fossa lesions, especially those that carry a high risk of morbidity with surgical treatment. The modern-day neurosurgeon has an excellent understanding of the complex anatomy of the posterior fossa, diagnostic modalities that facilitate the exact localization of lesions, and technological tools to supplement surgical skills. All of these advances have resulted in a dramatic reduction in morbidity and mortality. The ongoing evolution of treatment modalities and surgical techniques will continue to improve the care of patients with posterior fossa pathology. Fig. 1.10 Illustrations demonstrating the technique used by Dandy to remove acoustic neuromas. The capsule was enucleated (A) and then completely removed (B). 1. Green RE. Surgery of the posterior fossa. In: Waler AE, ed. A History of Neurological Surgery. New York: Hafner, 1967:114–133 2. Macewen W. Meningitis, abscess of the brain, infective sinus thrombosis. In: Pyogenic Infective Diseases of the Brain and Spinal Cord. Glasgow: J. Maclehose & Sons, 1893:354 3. Starr MA. Brain Surgery. New York: William Wood, 1893:295 4. Goodrich JT. Landmarks in the history of neurosurgery. In: Rengachary SS, Ellenbogen R, eds. Principles of Neurosurgery, 2nd ed. New York: Elsevier Mosby, 2005 5. Garofalo IG. Procedimenti Anatomici. Milano: Rizzoli, 1991 6. Frazier CH. The midline bloodless approach to the posterior fossa. Trans Am Surg Assoc 1926;44:229–247 7. Goodrich JT. Historical antecedents of skull base surgery. In: Janecka IP, Tiedemann K, eds. Skull Base Surgery, Anatomy, Biology and Technology. New York: Lippincott-Raven, 1996:17–54 8. Arasse D. Leonardo Da Vinci. New York: Konecky and Konecky, 1998 9. Dryander J. Anatomae, Hoc Est, Corporis Humani Dissectionis Pars prior Marpurgi, Apud E. Cervicornum. 1537 10. Hopstock H. Leonardo as an anatomist. In: Singer C, ed. Studies in the History of Medicine. Oxford: Clarendon Press, 1921:153–191 11. Leonardo RA. History of Surgery. New York: Froben Press, 1943:504 12. Hanigan WC, Ragen W, Foster R. Dryander of Marburg and the first textbook of neuroanatomy. Neurosurgery 1990;26:489–498 PubMed 13. Saunders JB, O’Mallery CD. The Illustrations from the Works of Andreas Vesalius of Brussels. New York: Dover, 1973 14. Vesalius A. De Humani Corporis Fabrica Libri Septum. Basel: Ex Officina Joannis Oporini, 1543 15. Singer C. Vesalius on the Human Brain. Introduction, Translation of Text. London: Oxford University Press, 1952;61 16. Hammond WA. A Treatise on the Diseases of the Nervous System, 7th ed. New York: D. Appleton, 1871:929 17. Rengachary SS, Xavier A, Manjila S, et al. The legendary contributions of Thomas Willis (1621-1675): the arterial circle and beyond. J Neurosurg 2008;109:765–775 PubMed 18. Stewart TG, Holmes G. Symptomatology of cerebellar tumors, a study of 40 cases. Brain 1904;27:522–591 19. Dandy WE. Localization or elimination of cerebral tumors by ventriculography. Surg Gynecol Obstet 1920;30:329–342 20. Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg 1918;68:5–11 PubMed 21. Lumsden T. The regulation of respiration: Part I. J Physiol 1923;58:81– 91, 111–126 PubMed 22. Dandy WE. Surgery of the brain. In: Lewis’s Practice of Surgery. Hagerstown, MD: WF Prior, 1945:6–7 23. Dandy WE. An operation for the total extirpation of cerebellopontine tumors in the cerebellopontine angle. A preliminary report. Bull Johns Hopkins Hosp 1922;33:344–345 24. Cairns H. Acoustic neurinoma of right cerebello-pontine angle. Complete removal. Spontaneous recovery from post-operative facial palsy. Proc R Soc Med 1931;25:35–40 25. Horsley V. On the technique of operations on the central nervous system. BMJ 1906;2:411–423 26. Krause F. Surgery of the Brain and Spinal Cord Based on Personal Experiences. Transl. by H. Haubold and M. Thorek. New York: Rebman, 1909: 820–1201 27. Ballance CA. Some Points in the Surgery of the Brain and Its Membranes. London: Macmillan, 1907:405 28. Naffziger HC. Brain surgery with special reference to exposure of the brain stem and posterior fossa, the principle of intracranial decompression, and the relief of impactions in the posterior fossa. Surg Gynecol Obstet 1928;46:240–248 29. Cushing H. Intracranial Tumors. Notes Upon a Series of Two Thousand Verified Cases with Surgical Mortality Percentages Pertaining Thereto. Springfield, IL: Charles C. Thomas, 1932:150 30. Cushing H. Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontine Angle. Philadelphia & London: WB Saunders, 1917: 296 31. Panse R. Ein Gliom des Akustikus. Arch F Ohrenh 1904;61:251–255 32. Frazier CH. Fifty years of neurosurgery. Arch Neurol Psychiatry 1935; 34:907–922 33. Dandy WE. An operation for the total extirpation of cerebellopontine tumors. Surg Gynecol Obstet 1925;41:129–148 34. Bliss M. Harvey Cushing, A Life in Surgery. New York: Oxford University Press, 2005 35. Frazier CH. Remarks upon the surgical aspects of tumors of the cerebellum. N Y State J Med 1905;18:272–280, 332–337
Surgery of the Posterior Cranial Fossa: Historical Aspects
♦ Galen

♦ Advances in Neuroanatomy and Neurophysiology
♦ Cerebellar Surgery
♦ Cerebellopontine Angle Tumors
♦ Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








