CHAPTER 389 Surgical and Radiosurgical Management of Giant Arteriovenous Malformations
Successful treatment of arteriovenous malformations (AVMs) remains one of the more challenging problems faced by neurosurgeons. Giant AVMs represent a rare, but excessively difficult group of AVMs that are often associated with higher treatment morbidity and mortality than their smaller counterparts. The standard definition of a giant AVM is a high-flow, angiographically visible vascular malformation that is greater than 6 cm in maximum diameter. The size alone results in a Spetzler-Martin grade of at least III, and most of these giant AVMs are classified as Spetzler-Martin grade IV or V.1 The size of these giant AVMs almost invariably results in at least a portion of the malformations being located within or immediately adjacent to eloquent regions of the brain, and these lesions often have both deep and superficial venous drainage. Giant AVMs frequently have an arterial supply from multiple vascular distributions, from the anterior as well as the posterior circulation, and in many cases the arterial supply is bilateral.
Clinical Findings/Preoperative Evaluation
Symptoms
The size of giant AVMs and the large amount of arteriovenous shunting within these lesions often result in symptoms unique from those with smaller AVMs. Small vascular malformations are typically manifested as headaches,2,3 seizures,4–6 or hemorrhage.5–9 Giant AVMs can have any of these symptoms but can also cause transient and progressive neurological dysfunction through cerebrovascular “steal.” The large blood volume shunting through these malformations can result in relative hypoperfusion in the surrounding neurological tissue with subsequent ischemia.10–13 This is due to lower blood pressure within the arterial feeders of the AVM than in the surrounding brain, which results in preferential shunting of blood to the AVM away from normal brain tissue.
AVMs have initial hemorrhage rates of 2% to 4% and annual rebleeding rates of approximately 4% to 18%.7,14–17 Mortality from each hemorrhage approximates 10% to 15%,5,8 and up to 50% of patients suffer some form of neurological deficit as a result of bleeding from an AVM.9 Some authors believe that larger AVMs have bleeding rates slightly lower than their smaller counterparts.7,18,19 Other studies have shown no consistent relationship between size and risk for hemorrhage,14,20 and feeding artery pressure (believed to be a risk factor for AVM hemorrhage) did not correlate with AVM size.21 However, giant AVMs with components in the basal ganglia, thalamus, and pineal region may be associated with not only a higher hemorrhage rate22 but also increased morbidity if they bleed, with more than 60% of these patients suffering significant morbidity or death from hemorrhage.23 Angiographic factors that have been shown to correlate with risk for AVM hemorrhage are central venous drainage, periventricular location, and intranidal aneurysms,24,25 as well as a single draining vein25 and stenotic venous drainage.26 By their size alone, giant AVMs are more likely to have a component of central venous drainage and a portion of the AVM adjacent to or within the ventricle.
Indications for and Contraindications to Surgery
Indications for resection of giant AVMs must consider both the natural history of the AVM and the combined risks associated with multimodality treatment for a particular patient.27,28 Most angiographically documented giant AVMs are candidates for treatment, particularly if they have hemorrhaged or are causing significant progressive neurological deficits, disabling headaches, or medically intractable seizures. The risk with surgical resection is generally related to AVM size and location and the complexity of arterial feeders and venous drainage,1 factors that make these giant AVMs a higher risk than smaller AVMs to treat with surgery alone. Larger lesions may require staged surgical resection or multimodality therapy combining microsurgery, embolization, and stereotactic radiosurgery.27–30 Other factors affecting treatment risk include the patient’s clinical condition and age and surgeon experience.
The location of the giant AVM under consideration significantly affects the risk associated with surgical resection.27–32 Because of their size, these larger AVMs often involve eloquent cortex, which significantly increases the risk of resection. Resection of giant cortical AVMs may cause motor or sensory deficits (frontal/parietal) or visual field defects (occipital), memory deficits, and changes in cognition and personality (cingulate gyrus and corpus callosum). Treatment of deep portions of giant AVMs within the basal ganglia and thalamus can result in hemiplegia, sensory deficits, dysphasia, and cognitive and memory deficits.23,33,34 The clinical condition of the patient also has a bearing on surgical resection, with poor-grade patients usually resulting in a poor outcome.29 Younger patients have a better benefit-to-risk ratio for the treatment of giant AVMs for two reasons. First, the brain of a young patient can better tolerate resection of the vascular malformation and has improved recovery after surgery.35 Second, a younger patient translates into longer AVM-free survival on completion of resection.35 Likewise, patients with a significant mass effect from a hemorrhagic clot resulting from AVM rupture should undergo emergency evacuation of the hemorrhage, with resection of the AVM being reserved for a later date. The timing of resection of giant AVMs is similar to that for other AVMs in that resection should be planned under nonemergency conditions to minimize patient morbidity. Resection of the AVM 4 to 6 weeks after hemorrhage, when the blood is liquefied, may improve the ease of resection.8,23,30,36 In many cases, the hematoma cavity has performed a portion of the dissection around the nidus of the AVM. Furthermore, this short interval allows the patient to achieve stabilization or improvement of any neurological deficits.
Failure of stereotactic radiosurgery and embolization is another indication for surgical resection,27,30,32 although in the case of giant AVMs, these treatment modalities are generally used as adjuncts in reducing AVM size before planned microsurgical resection. Most studies indicate that partial obliteration after radiosurgery does not confer protection from AVM rehemorrhage,23,27,29,33,37,38 and hence an alternative therapy should be sought for residual AVMs seen more than 3 years after radiosurgical treatment. Embolization is a useful treatment of giant cortical AVMs,39 but it is less useful in the treatment of deeper arterial feeders (thalamoperforating, choroidal, and lenticulostriate arteries) within the basal ganglia and thalamus. These vessels primarily arise from the parent vessels at right angles, have small lumens, and are thus difficult to canulate.23,33,34 Nonetheless, in selected cases, embolization has been extremely helpful in partially obliterating even basal ganglia and thalamic arterial feeders.29,30,40
Hemodynamics Of Giant Arteriovenous Malformations
All AVMs serve as a shunt between the arterial and venous systems. Pressure within the arterial feeders of AVMs has been shown to be lower than the pressure in normal brain arteries, whereas pressure within the venous channels draining the AVM has been shown to be higher than normal venous pressure. Furthermore, as a result of this low pressure on the arterial side, blood that would normally supply the cortex adjacent to the AVM may preferentially flow to the AVM. This cerebrovascular steal, which may be more pronounced with giant AVMs, is hypothesized to cause ischemic symptoms within the cortex adjacent to the AVM. The arteries within the adjacent cortex respond to this steal phenomenon by dilating their lumen to maintain flow through autoregulation. The extended interval in which these cortical arteries remain dilated results in the loss of their rapid ability to constrict should the blood pressure within these cortical arteries change. When these vessels are subjected to a higher pressure head, as when the low-resistance high-flow giant AVM is removed, they may not have adequate autoregulatory compensation, and edema and hemorrhage in the surrounding brain parenchyma can result. Angiographic characteristics found to be correlated with cerebrovascular steal are angiomatous change, AVM size, and pattern of peripheral venous drainage.41
The process of impaired autoregulation and hemorrhage from cortical vessels adjacent to an AVM after AVM resection is called normal perfusion pressure breakthrough.42 Although this phenomenon is rare, it is believed to occur more frequently in giant AVMs because these malformations shunt a higher volume of blood and thus create more vascular steal from the surrounding cortex.27 AVM features associated with an increased likelihood of normal perfusion pressure breakthrough (other than AVM size) include dilated feeding arteries, poor filling of the surrounding cerebral vasculature on angiograms, clinical symptoms of ischemia from cerebral vascular steal, and physiologic evidence of impaired autoregulation in the brain.31,43 Normal perfusion pressure breakthrough–associated bleeding can be limited by careful control of blood pressure in the immediate postoperative period, staging of resection of the giant AVM, and the judicious use of presurgical embolization or radiosurgery, or both, in an attempt to reduce the size of the arteriovenous shunt.
Evaluation
Angiography
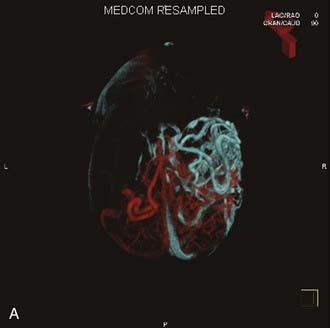
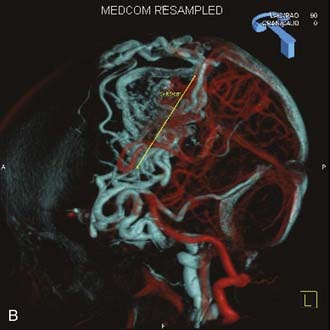
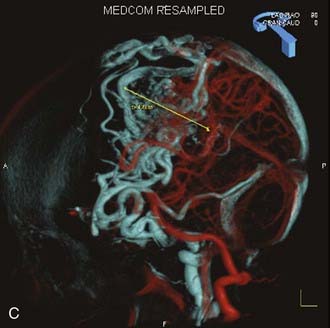
Angiography remains the “gold standard” with respect to evaluation of AVMs. Because of the multiplicity of arterial feeders to these giant AVMs, it is important that a four-vessel angiogram be performed to document all of the arterial supply. Giant AVMs may have a portion of their blood supply from extracranial sources, so both external carotid arteries should be evaluated.44 As with smaller AVMs, multiple views should be obtained to ensure that the neurosurgeon has a complete understanding of the origin of all the arterial feeders, the size and configuration of the AVM, and the number and direction of veins draining the AVM. A complete initial angiogram also allows better comparison with subsequent angiograms obtained during and after treatment. Angiography can also potentially document patients with a substantial amount of cerebrovascular steal as a result of the giant AVM. In these cases, normal cortical vessels may show more limited flow than other vessels at a distance from the AVM. In addition to standard two-dimensional angiography, the more recent development of three-dimensional angiography has improved the ability to identify the true size of the AVM (Fig. 389-1).45
Post-treatment angiograms should be performed to ensure complete obliteration of the AVM, either soon after microsurgical resection of the final portion of the giant AVM or within several years after stereotactic radiosurgery if this modality is used as the final treatment within a staged approach to a giant AVM. It is critical to confirm complete treatment of an AVM, particularly giant AVMs, because leaving a residual AVM component is more problematic and reports suggest that partial treatment does not protect against future hemorrhage.37,38,46–49
Angiography also plays an important role in the treatment of AVMs with stereotactic radiosurgery. Current radiosurgical systems rely on a fusion of angiography and magnetic resonance imaging (MRI) or computed tomography (CT) as a basis for planning treatment. In older radiosurgical systems, angiography is performed with a stereotactic base ring (or bite block) attached to the patient’s head and a fiducial cage mounted to the base ring. The fusion of angiography and MRI/CT allows improved targeting of the nidus while avoiding extraneous radiation doses to the draining veins or the surrounding cortical tissue. Newer image-guided radiosurgery is performed without a stereotactic ring, and in these cases, three-dimensional angiographic images can be directly fused to stereotactic CT and MRI during the treatment-planning process.45
Magnetic Resonance Imaging
Although angiography remain the primary modality for evaluation of giant AVMs, pretreatment MRI provides additional information. MRI shows the relationship of the giant AVM to other intracranial structures, thereby affording the neurosurgeon a better understanding of the anatomy surrounding the AVM. The size and direction of large draining veins can often be noted, as can evidence of previous hemorrhage. MRI is also critical during the planning of stereotactic radiosurgery in conjunction with cerebral angiography. In select cases of giant AVMs with irregular shapes or deep components, intraoperative navigation with MRI-based guidance can be extremely useful. More recent developments in MRI technology have allowed the incorporation of three-dimensional imaging to assess both the AVM nidus itself50–53 and white matter tracts adjacent to the AVM.54–56 Functional MRI has also been used to elucidate the relationship between an AVM and eloquent areas of the brain.57
Xenon Computed Tomography
Xenon CT is rarely used in the evaluation of smaller AVMs, but for larger AVMs with significant surrounding cortical ischemia as a result of steal, this modality may be useful in determining the degree and distribution of hypoperfusion.58 Impaired augmentation indicates failure of autoregulation and may identify patients at high risk for normal perfusion pressure breakthrough bleeding.
Treatment
Because of the relative infrequency of giant AVMs, which are thought to account for 10% of all AVMs,14 and the complexity of arterial feeders and venous drainage, treatment is individualized for each patient. It is rare for these giant AVMs to be treated successfully at a single sitting with one modality, and the best patient outcomes often involve staged treatment with two or more of the treatment modalities available.
Embolization
Because giant AVMs are difficult to treat with microsurgery alone and are too large to be optimal targets for stereotactic radiosurgery, embolization is generally the first course of treatment. Historically, this process consists of using either small polyvinyl alcohol particles31 or cyanoacrylate glue59–62 in an attempt to occlude the feeding arteries. Over the past decade, n-butyl-2-cyanoacrylate (NBCA) has emerged as the main embolic agent of choice because its soft nature after occluding portions of the nidus does not interfere with later surgical resection of the AVM.61 Recently, ethylene vinyl alcohol copolymer (Onyx, Micro Therapnetics, Irvine, CA) has been studied as an embolic agent, with several clinical series documenting the efficacy of this agent.63–67 The primary goal of embolization is to reduce volume and flow in the AVM to facilitate safer and more effective subsequent treatment with microsurgery or radiosurgery (or both). Embolization of deep feeders to the AVM or deep AVM components may help in later surgical resection. Occasionally, embolization alone is used for palliation of symptoms from giant AVMs with no intent of obliterating the entire lesion. Embolization is usually performed in several stages, often three or more, for giant AVMs.44 Staged embolization allows the AVMs to adjust to changes in flow hemodynamics after each embolic treatment; attempts to embolize large portions of a giant AVM in a single stage are frequently associated with increased risk for hemorrhage. Giant AVMs can have a significant external carotid component, and embolization of these vessels often makes the craniotomy opening easier for the surgeon, as well as reduces blood supply to the AVM. Many giant AVMs have arterial supplies from both the anterior and posterior circulation and frequently from both hemispheres, and therefore multiple vessels need to be cannulated in an attempt to achieve optimal embolization. Care must be taken to avoid passing embolic glue or particles into normal cortical vessels to avoid the development of infarcts.
During embolization, patients with giant AVMs are monitored with electrophysiologic potentials, which provides several benefits.39,40 First, such monitoring is particularly useful in identifying when normal cortical vessels are inadvertently occluded. Second, electrophysiologic monitoring during embolization can also provide some insight to the neurosurgeon regarding whether the patient’s brain can tolerate periods of relative hypotension (which may be used later during surgery to reduce bleeding). This is particularly relevant when treating an AVM with “steal” or ischemic symptoms. Furthermore, when a particular vessel is in question, amobarbital sodium (Amytal) can be injected into the vessel before embolization to determine whether the vessel is supplying an area of eloquent cortex and hence would cause a neurological deficit if occluded.68
Some institutions use intraoperative embolization extensively in treating giant AVMs.31 The advantages of this technique are that the risk of reflux into normal vessels is decreased and the surgeon has the opportunity to inject numerous distal vessels inaccessible to the neurointerventionalist in the angiography suite. Intraoperative embolization proceeds once the surgeon has performed the craniotomy and exposed the AVM. Injection of the embolic agent should be performed as close to the AVM nidus as possible to minimize reflux into normal vessels. After temporary occlusion of the proximal portion of the vessel, 27-gauge needles can be introduced and several cubic centimeters of NBCA injected. This process can be repeated for various feeding arteries until flow to the AVM is significantly reduced. After embolization, each feeding artery can be sacrificed with either a small clip or bipolar coagulation.
Stereotactic Radiosurgery
Stereotactic radiosurgery has established itself as successful treatment of certain intracranial AVMs, particularly small or moderate-sized AVMs located in critical brain regions. A number of clinical series using either heavy charged particles (protons and helium ions) or photons (Gamma Knife or linear accelerator) have demonstrated that AVMs less than 3 cm in diameter treated with 20 to 25 Gy equivalents (GyE) have a 3-year obliteration rate of 80% to 95% with a low complication rate (2.5% to 4.5% permanent neurological deficits, 2.5% to 4.5% transient deficits).29,37,38,69–77 However, various limitations of stereotactic focused radiosurgery have become apparent after analyzing the results in treating AVMs larger than 3 cm. These AVMs have a much lower obliteration rate, even after 3 years (33% to 58%), and a higher complication rate (20% to 30%) with treatment doses of 15 to 20 Gy.29,38,70,73,75–79
The primary mechanism of AVM obliteration after radiosurgery involves hyperplasia of the vascular intima80 with progressive narrowing and ultimately occlusion. Such obliteration typically takes place over a 2- to 3-year period. There are three considerations when weighing surgery versus radiosurgery for the treatment of AVMs. First, AVMs larger than 4 cm in diameter have only a 33% to 50% rate of obliteration at 3 years after radiosurgery but a 20% to 30% complication rate with treatment doses of 15 to 20 Gy.38,77 The rate of obliteration is increased for larger AVMs when higher doses are used (25 to 45 Gy); however, the risk for radiation-induced complications increases significantly. Second, the risk for intracranial hemorrhage persists during the interval between treatment and complete obliteration.38,75,77 Third, serial radiographic studies, including cerebral angiography, are necessary to confirm complete obliteration.
Despite these limitations, radiosurgery may be associated with less morbidity than surgical resection in patients with portions of giant AVMs in eloquent brain.28,30 Radiosurgery can be used as a preoperative adjunct to obliterate portions of giant AVMs and thus decrease the size of the remaining nidus for later surgical resection.29 Some authors have recommended staged stereotactic radiosurgery when treating giant AVMs.81 In these cases, multiple radiosurgical treatments are delivered to different portions of the AVM at various intervals to avoid delivering treatment to a single large target. This theoretically reduces the risk for radiation necrosis.
Some patients with giant AVMs require more than one course of stereotactic radiosurgery, and this option has been used in selected patients.29,82 The disadvantages of such an approach are the second latency period of 1 to 3 years before obliteration occurs, the possibility that a second radiosurgical treatment may still not obliterate the AVM, and the risk for radiation-induced injury, which may be higher with a second radiosurgical treatment.
Microsurgery
Another indication for surgical treatment of giant AVMs would involve resection of radiation necrosis. Radiation injury after stereotactic radiosurgery may result in significant mass effect and edema and require prolonged treatment with high doses of corticosteroids. We have observed several patients with steroid dependence as a result of radiation injury who were successfully tapered off corticosteroids after resecting areas of necrosis, as well as some patients who have shown substantial neurological improvement.83
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree