CHAPTER 362 Surgical Decision Making for the Treatment of Intracranial Aneurysms
Overview: The Status of the Patient and the Natural History of the Aneurysm
The status of the patient and the natural history of the aneurysm must be considered together. Many patient-related factors will determine outcome irrespective of the technical treatment of the aneurysm. Choosing the treatment modality that is safest and most efficient for each individual patient is an important therapeutic decision. In addition, a variety of factors such as whether the aneurysm is intact or ruptured, aneurysm size and location, the patient’s age and medical condition, and associated factors such as intracerebral hemorrhage (ICH), intraventricular hemorrhage (IVH), and clinical grade after aneurysm rupture can all influence the outcome of attempted aneurysm occlusion. It is important therefore to consider these various factors before treating each patient. Taken together, the natural history of the patient and the natural history of the particular aneurysm must be considered in evaluating the overall approach to treatment. For example, the treatment of a small basilar tip aneurysm in a 40-year-old and in an 80-year-old will differ despite the similarity in the lesion. Thus exclusive focus on either the patient or the aneurysm is not an optimal management strategy. Patient considerations and the natural history of cerebral aneurysms are comprehensively reviewed in Chapter 354 and Chapter 360.
Neuroradiological Evaluation
These studies include computed tomography (CT), a variety of magnetic resonance imaging (MRI) sequences, CT angiography (CTA), MR angiography (MRA), and cerebral angiography. Digital subtraction angiography (DSA) is the current “gold standard” to evaluate aneurysms. Noninvasive techniques are evolving rapidly and CTA in particular frequently can provide a useful complement to DSA. A combination of imaging techniques often is necessary, particularly for more complex aneurysms; this permits accurate preoperative planning and a full understanding of the relevant anatomy and these studies will often be critical in determining timing (e.g., presence of intracerebral clot in a poor grade patient), selection of treatment options (e.g., endovascular versus open surgery) and surgical approaches (e.g., low-versus high-lying basilar tip aneurysm; atretic A-1 in an anterior communicating aneurysm). For surgical planning, CTA with three-dimensional reconstructions and spin, rotational, and three-dimensional DSA (3D-DSA) data in an orientation that reproduces the neurosurgeon’s intraoperative view is a valuable adjunct.1 This is particularly useful for large and complex aneurysms. The following features need to be evaluated: (1) the aneurysm’s parent vessel; (2) aneurysm size, shape, and relationship to parent and adjacent arteries; (3) the presence and location of vasospasm; (4) adjacent vessel displacement consistent with mass effect (e.g., partial aneurysm thrombosis [i.e., the aneurysm is larger than that seen on DSA]); and (5) the presence of other aneurysms or vascular abnormalities. The reader should consult Chapter 359 and Chapter 360 for more detailed information.
Unruptured and Ruptured Aneurysms: Impact on Decision Making
Unruptured Aneurysm
Efficacy of Treatment
There are few studies that evaluate the success rate of surgery in achieving complete occlusion of unruptured aneurysms and the long-term subsequent angiographic reoccurrence or SAH. Tsutsumi and coworkers2 followed 115 patients for almost 9 years after their unruptured aneurysm had been clipped and found only 1 patient (0.8% over 8.8 years or <0.01% per year) suffered a SAH from regrowth of the original aneurysm. Most studies are focused on patients initially having a SAH or a mixed population of both intact and ruptured aneurysm patients. Extrapolation from surgical series after a SAH suggests that the complete occlusion rate is high and that such occlusion is not perfect, but protective (see discussion later).
Surgical Risk
Surgical outcome after repair of unruptured aneurysms has been described in several case series dealing with unruptured aneurysms. Most case series3–9 demonstrate that the morbidity and mortality associated with surgical treatment of unruptured aneurysms is very low; about 5% of patients die or are disabled. By contrast, findings from the International Study of Unruptured Intracranial Aneurysms (ISUIA), a prospective study including 1172 patients, 85% of whom underwent surgery, suggested that overall surgical risk was much higher; the combined morbidity and mortality approached 15% at 1 year.10 In particular, surgical risk was greater than the risk of hemorrhage among patients with an incidental aneurysm less than 10 mm in diameter. In ISUIA II in-hospital mortality was 1.8% and adverse outcomes were observed in 8.9% of survivors.11 Administrative discharge data from New York and California suggest mortality is between 2.5% and 3% and adverse events occur in 21.3% to 22.4% of patients.12,13
Several authors have performed meta-analyses of surgical series to clarify surgical risk14; the majority suggest that the combined morbidity and mortality is between 3% and 7%. For example, King and associates14 performed a meta-analysis of 28 clinical series containing 733 patients; overall, 4.1% of patients were disabled and 1% died. Similarly, Raaymakers and coworkers15 performed a meta-analysis from a Medline search between 1966 and 1996 and identified 61 studies involving 2460 patients. Surgical morbidity was 10.9% and mortality 2.6%; the risk of a poor outcome decreased in more recent years. It is difficult, however, to derive meaningful conclusions by combining the various surgical series for several reasons: (1) most are reported by surgeons with an inherent bias; (2) unruptured aneurysms of various sizes and location are included in each series; (3) the technique of aneurysm occlusion, such as wrapping or clipping, is not always described; (4) the effectiveness of surgery is rarely described because postoperative angiography is rarely performed; (5) description of outcome measures is lacking; and (6) neuropsychological and quality of life assessment is lacking. It must be recognized that much of the data on results of aneurysm surgery also come from high volume centers and single-center studies, and publication bias in particular must be considered when applying the reported risks of aneurysm surgery to a broader population.16 In addition administrative data often describe a discharge condition rather than a 3- or 6-month outcome.
Factors That Are Associated with Surgical Outcome
Increased aneurysm size is the most important factor associated with surgical complications and poor outcome (Fig. 362-1).7,17,18 Overall, aneurysm size greater than 25 mm has a fourfold increased risk compared with a 5-mm aneurysm.19 For example, Wirth and associates8 demonstrated that significant operative morbidity complicated surgery in 2% of unruptured aneurysms less than 5 mm, 7% of aneurysms between 6 and 15 mm, and 14% of aneurysms greater than 16 mm in diameter. Similarly, Solomon and coworkers7 observed a favorable outcome in 83% of patients who underwent surgical repair of giant unruptured aneurysms. By contrast, 99% of patients who had aneurysms less than 10 mm in diameter experienced good outcomes. The association between increased aneurysm size and poor outcome may be explained, in part, by the aneurysm’s intimate association with small perforators, the broad aneurysm neck, intraluminal thrombosis, or atherosclerosis in the aneurysm neck or dome.
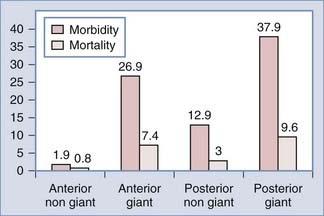
FIGURE 362-1 Surgical risk for unruptured intracranial aneurysms.
(The data are from 61 studies including 2460 patients treated between 1966 and 1996 and reviewed in Raaymakers TW, Rinkel G, Limburg M, and coworkers. Mortality and morbidity of surgery for unruptured intracranial aneurysms. A meta-analysis. Stroke. 1998;29:1531-1538.)
Repair of unruptured posterior circulation aneurysms, particularly giant basilar bifurcation aneurysms, is associated with increased surgical risk (Fig. 362-1).15,19 For example, Solomon and associates7 observed that giant unruptured basilar aneurysms were associated with 50% surgical morbidity and mortality. By contrast, the morbidity and mortality for giant anterior circulation unruptured aneurysms was 13%. Reasonable surgical results can be expected with nongiant unruptured posterior circulation aneurysms.6 However, when compared with similar size lesions in the anterior circulation, there is a nearly tenfold increase in risk.19,20 In the anterior circulation, anterior communicating artery and ICA bifurcation lesions appear to have the greatest risk.8
Several other aneurysm features identified on angiogram such as aneurysm orientation, wide aneurysm neck, atherosclerosis, and calcification in the aneurysm neck are associated with increased risk. For example, we have observed a significant increase in surgical complications among posterior oriented basilar bifurcation aneurysms.21 Similarly, there is an increased risk among posterior superior oriented anterior communicating artery aneurysms. Calcification in the aneurysm neck often is associated with poor outcome, in part, because many of these lesions are large or giant aneurysms. In addition, multiple clips frequently are necessary to occlude the aneurysm, leading to an increased incidence of cerebral embolism. Calcification may be removed by endarterectomy; despite this, surgical results remain poor because the remaining wall for clip placement is often very friable and thin. Preoperative CT scanning can help identify neck calcification. Hypothermic circulatory arrest should be considered in the reconstruction of some heavily calcified aneurysms.
There are several patient-related factors including advanced age, ischemic cerebrovascular disease, and medical conditions such as diabetes mellitus that increase the risk of unruptured aneurysm surgery.7,8,17,19 A recent retrospective, single-center, multivariate analysis by Ogilvy and coworkers demonstrated that after aneurysm size and location, patient age is the next most important risk factor of poor outcome.18 In addition, in some patients such as those with severe cardiac or pulmonary disease, the anesthetic risk of surgery may carry a great immediate risk whereas in others, such as patients with advanced malignancy, any potential benefit is negated by a reduced life expectancy.
Which Patient with an Unruptured Aneurysm Should Be Treated?
In 1994 and 200922,23 the American Heart Association published guidelines for the treatment of unruptured intracranial aneurysms. Although there are no randomized trials, the consensus reports recommended occlusion of unruptured aneurysms greater than 5 to 7 mm among patients with acceptable surgical risk.
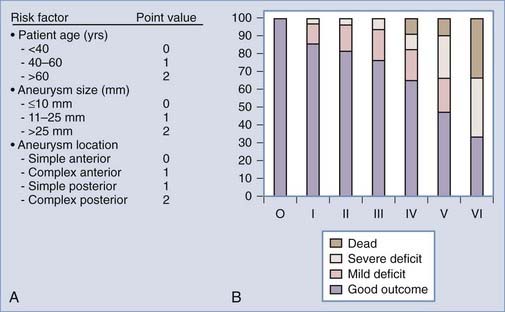
Since then several studies have improved our understanding of the natural history and surgical risk and alternate treatments for unruptured aneurysms have evolved. Management should be tailored to the patient’s risk and the physician’s own experience. Microsurgical, endovascular, and alternative strategies such as vessel occlusion with or without bypass or no treatment other than observation and clinical follow-up should be considered, specific to the patient and the aneurysm. Khanna and associates19 have attempted to do this by creating a grading scale for unruptured aneurysms (Fig. 362-2). This scale was derived from a retrospective analysis of 172 patients who underwent treatment of unruptured aneurysms and then validated prospectively in 50 patients. The incidence of poor outcome progressively increased from 0% in grade 0 to 66% in grade IV patients. Garrett and associates24 recently performed a comprehensive literature review (10,545 patients) and using mathematical models created an online open access program to quantify the benefit of surgery on unruptured aneurysms according to age, size, and location. Small basilar and supraclinoid aneurysms (5 mm) only had an operative benefit in young patients (40 years). By contrast, larger aneurysms (10 to 15 mm) had an operative benefit for a wider age range. There was no age and size combination that provided an operative benefit for cavernous aneurysms.
The case fatality rate for aneurysmal SAH remains high,25 and it is recognized that the main determinant of outcome is the severity of the initial bleed. It is therefore postulated that if SAH could be prevented before aneurysm rupture, poor outcomes theoretically may be avoided. However, because few asymptomatic aneurysms actually rupture and because all aneurysm treatment carries some risk, the management of patients with an unruptured aneurysm remains controversial.22 Based on the available literature, we recommend the following patients with unruptured aneurysms should be treated: (1) SAH from another aneurysm, (2) symptomatic aneurysm, (3) aneurysms more than 7 to 10 mm in nearly all patients with a life expectancy of 12 or more years, and (4) aneurysms more than 5 mm if the patient is young or middle-aged. These recommendations depend on collaboration between a highly experienced cerebrovascular team of microneurosurgeons and endovascular neurosurgeons at a tertiary medical center with a high case volume. Small, incidental aneurysms less than 5 mm in diameter should be managed conservatively in most cases; the exception is when there is a positive family history. In addition patients who smoke and are hypertensive may be considered for treatment when the aneurysm is small. In select patients when both treatment and natural history carry very high risks (e.g., those with giant aneurysms), nonoperative management also may be elected. When aneurysm occlusion is not recommended, patients should be counseled about risk factors and serial imaging (CTA or MRA) used to follow the aneurysm because an increase in size or a morphology change appears to represent an indication for treatment. For a small aneurysm (e.g., 4 mm) a 1-mm change in diameter represents a doubling of the aneurysm volume. This size change, however, may be difficult to detect and it is not precisely defined whether growth always proceeds rupture. In addition, how frequently imaging is required is not clear. Consequently recommendation of serial imaging is somewhat controversial (we obtain serial noninvasive imaging every 6 months).
The Ruptured Aneurysm
Aneurysm Rebleeding
Rebleeding is a major cause of poor outcome after SAH and is currently the most treatable cause of poor outcomes and will be a determinate factor in the timing of treatment (see later discussion). Approximately 70% to 90% of patients who rebleed die. Untreated, between 20% and 30% of aneurysms rerupture within the first 30 days and then at a rate of approximately 3% to 5% per year.26 The risk of rebleeding is greatest on day 1 (4%)—it may also complicate about 10% of patients in the ambulance or at the referring hospital before admission to the treating hospital.27 Recent evidence suggests that the risk of “ultra early rebleeding” (within 24 hours of SAH) may be as high as 15%.27,28 Rebleeding then occurs at a constant rate of between 1% and 2% per day during the subsequent 4 weeks.29,30 Poor clinical grade, abnormal hemostatic parameters, higher rates of intracerebral hematoma or intraventricular hematoma and posterior circulation aneurysms appear to be associated with a greater risk of rebleeding.27,31,32 Some studies suggest that ventricular drainage may be associated with rebleeding; however, when preoperative ventriculostomy is followed by early treatment of the ruptured aneurysm, the rebleeding risk is not increased by the ventriculostomy.33 Blood pressure must be monitored and controlled; however, the relationship of elevated blood pressure to rebleeding is not well elucidated, and control of hypertension must be balanced against the risk of cerebral infarction.22,27,32,34 The major goal in SAH treatment is aneurysm obliteration to prevent rebleeding. Aneurysm rebleeding may be reduced by antifibrinolytic administration, but can only be prevented by direct obliteration using surgical or endovascular techniques. No well-controlled studies exist that answer whether blood pressure control in acute SAH influences rebleeding. There are two important questions: when should surgery be performed and which patients should undergo surgical or endovascular aneurysm occlusion?
Timing of Aneurysm Obliteration
Early surgery eradicates the risk of rebleeding and appears to be associated with improved outcome. For example, among the 722 patients treated at 27 North American centers in the International Cooperative Study on the Timing of Aneurysm Surgery, a prospective epidemiologic, but nonrandomized study found early surgery significantly improved outcome.35 Adjusted overall outcome demonstrated that 70.9% of patients undergoing surgery between 0 and 3 days after aneurysm rupture experienced a good recovery, whereas 62.9% of patients enjoyed a similar outcome if surgery was performed after 14 days. Brilstra and associates36 examined how effective aneurysm clipping was at reducing poor outcome associated with rebleeding: a risk reduction of 19% was observed in patients who had surgery rather than conservative management.
For each patient with a ruptured aneurysm, there are several factors, including aneurysm-related, hemorrhage-related, and patient-related factors, that influence whether to operate acutely or in a delayed fashion. Among patient-related factors, neurological grade and age are the most important. Poor grade patients are at greater risk for rebleeding and vasospasm.37,38 Early aneurysm occlusion should therefore be achieved. Although cerebral swelling is more frequent, early surgery in poor grade patients is not associated with a greater risk of surgical complications than in good grade patients.39 Alternatively, those patients who demonstrate significant swelling on admission CT scan may be excellent candidates for endovascular occlusion. Advanced age is associated with poor outcome; however, this should not exclude the patient from early surgery, in part because elderly patients are more likely to have intracerebral hematomas and a decreased cerebrovascular reserve that increases their risk for delayed ischemia.40 Similarly these patients may be excellent candidates for endovascular aneurysm occlusion. Early surgery may be useful in those patients at high risk for vasospasm, such as those with thick SAH on CT, in part because the role of hypervolemic therapy and angioplasty may be best when the aneurysm is secured. Delayed surgery may be preferable for complex lesions such as giant aneurysms or those where prolonged periods of temporary occlusion are expected to achieve aneurysm occlusion.
Several lines of evidence demonstrate that surgery within 3 days of SAH is associated with improved outcome among patients with ruptured anterior circulation aneurysms. For example, a single randomized study has addressed surgical timing in good clinical grade (Hunt and Hess I-III) patients following anterior circulation aneurysm rupture.41 At 3 months, independent outcomes were observed in 91.5% of patients undergoing surgery within 3 days, 78.6% between 4 and 7 days, and 80% undergoing surgery greater than 8 days after a SAH. Nonrandomized studies that describe concurrent cohorts or historical controls and large clinical series35,42–48 have observed a tendency for patients undergoing early surgery to experience better outcomes.
The timing of aneurysm obliteration for posterior circulation aneurysms is less well defined. In addition, because many of these patients now undergo endovascular procedures, the question may be less relevant. Most information that favored delayed surgery came from specialized referral centers. This may bias results because epidemiologic studies demonstrate that patients with posterior circulation aneurysms are 3 times more likely to die before reaching the hospital or within the first 48 hours of a SAH than patients with anterior circulation lesions.49–51 Several recent studies suggest that early surgery may reduce morbidity and mortality.52–54 For example, Hillman and associates53 prospectively reviewed 59 cases of ruptured posterior fossa aneurysms treated during 1 year. Fifty percent of patients scheduled for late surgery (n = 26) made a good recovery, whereas 72% scheduled for early surgery (n = 23) made a good recovery. Intraoperative complications were equally frequent in early or delayed surgery. When surgery is indicated rather than endovascular occlusion, we recommend early surgery for ruptured posterior circulation aneurysms except for those lesions that may present technical difficulty, such as large posterior-oriented basilar bifurcation aneurysms.
Earlier aneurysm occlusion using surgical or endovascular techniques has reduced the impact of rebleeding on outcome.32 In addition, early aneurysm occlusion permits more aggressive and early management of cerebral vasospasm with hemodynamic therapy and interventional management. Increased time to treatment, however, remains associated with increased rates of preoperative rebleeding. There is evidence that time from SAH to treatment is shorter in patients who have their aneurysm occluded with endovascular techniques. For example, in the International Subarachnoid Aneurysm Trial (ISAT), the mean time to treatment was 1.1 days for endovascular occlusion and 1.8 days for surgery. Consequently there were fewer preoperative rebleeds in the endovascular group.55,56 Some investigators28,57 advocate short-term antifibrinolytic therapy (e.g., ξ-aminocaproic acid [EACA]) started at diagnosis and administered for a maximum of 72 hours to reduce rebleeding while waiting for treatment.
Efficacy of Aneurysm Surgery
Aneurysm surgery is effective; overall more than 90% of aneurysms undergoing surgical clip occlusion are completely obliterated at surgery.39,58–62 For example, Feurerberg and associates60 retrospectively examined 715 patients operated on between 1970 and 1980. Twenty-seven patients (3.8%) had incomplete obliteration on follow-up angiography; only 1 patient rebled during 266 person-years of follow-up. David and associates59 observed that the rate of complete occlusion on postoperative angiography was 91.8%. The rate of aneurysm recurrence was 0.5% and there were no hemorrhages among completely occluded aneurysms. The annual hemorrhage rate was 1.9% among incompletely occluded aneurysms. However, there are very few studies that report the routine use of postoperative angiography, suggesting that many patients may not undergo postoperative evaluation. This may be important because several clinical series suggest that even when a surgeon believes the operative result is satisfactory, vessel occlusion or aneurysm remnants may be found on 5% of postoperative angiograms.61,63–65
What factors are associated with failure to occlude the aneurysm? Aneurysmal remnants and vessel occlusion are more likely when a large aneurysm or cerebrovascular atherosclerosis is identified or multiple attempts to place aneurysm clips are made.61 For example, Solomon and associates,7 describing the surgical treatment of 202 unruptured aneurysms, observed that increased aneurysm size is associated with poor technical results. Among giant aneurysms only 60% underwent clip occlusion at surgery. By contrast, clip occlusion was achieved in 85% of lesions greater than 10 mm and 93% of lesions less than 10 mm. Postoperative angiographs were obtained in two thirds of the patients; complete aneurysm occlusion was observed in greater than 90% of the small aneurysms but only 54% of the giant aneurysms. During surgery, inadequate brain relaxation is associated with poor technical results. For example, Giannotta and Litofsky66 retrospectively reviewed 524 patients, including 20 who required reoperation for inadequately treated aneurysms. Fourteen of the reoperations were attributed to failure to obtain a slack brain or inadequate bone exposure at the initial surgery.
The Poor Grade Patient
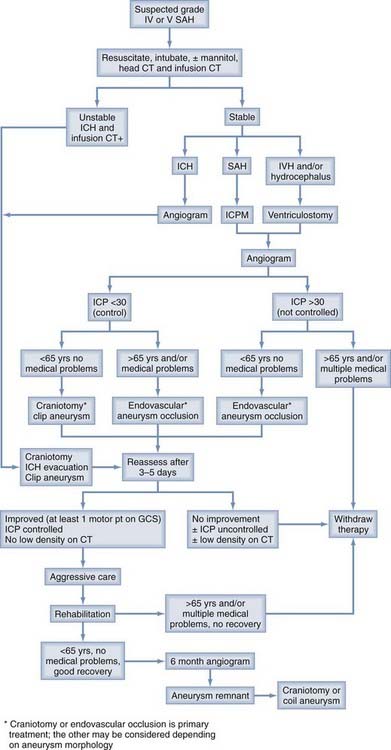
Between 20% and 40% of patients admitted to a hospital after aneurysm rupture are in poor clinical condition (Hunt and Hess grades IV and V). The association between poor outcome and poor clinical grade following a SAH is well described. However, the published data suggest that an aggressive policy may provide these patients their best chance of recovery (Fig. 362-3). This treatment includes rapid resuscitation and transport to a hospital, intracranial pressure (ICP) control, early aneurysm occlusion, prophylaxis against delayed cerebral ischemia, and care in a dedicated neurointensive care unit. In addition the use of noninvasive physiologic assessment such as transcranial Doppler, single photon emission computed tomography (SPECT), and invasive monitors for ICP and cardiac hemodynamics are necessary to guide therapy. Control of ICP is important25 and some poor grade patients may benefit from a decompressive craniectomy.67,68 Other monitors, such as microdialysis or brain oxygen offer promise but are still relatively clinically unproved.69 We routinely monitor brain oxygen tension (PbtO2) to help prevent brain hypoxia because this is associated with worse outcome.70 Epidemiologic studies demonstrate that up to 15% of the patients die before reaching the hospital and 30% die within the first 48 hours of aneurysm rupture.49,50,71,72 To be successful, therefore, an aggressive approach requires an organized multidisciplinary, proactive critical care approach that starts with in-the-field resuscitation and immediate ICP control. Alternate, but less successful, strategies include treatment of select patients, or delayed treatment when clinical improvement is observed.37,73,74
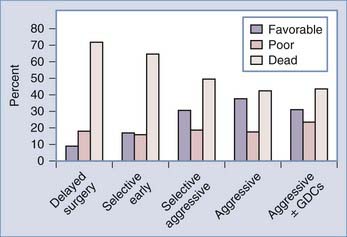
FIGURE 362-3 Overall management outcome for Hunt and Hess grade IV or V patients.
(Modified from Table 21-3 in Le Roux P, Winn HR. Management of the ruptured aneurysm. In: Le Roux P, Newell D, Winn HR, eds. Management of Cerebral Aneurysms. Philadelphia: Elsevier; 2004:303-333.)
Aggressive management requires a large commitment of resources; should all poor grade patients be treated? In the individual poor grade patient, admission clinical and radiographic findings are often insufficient to accurately predict outcome.37,73–77 For example, predicting outcome based only on admission findings may result in withholding treatment from 30% of the grade IV and V patients who subsequently experience favorable outcomes.74 Consequently we believe that management should be initiated in most patients. Continued observation, including an ICP monitor and follow-up CT scan, helps define outcome. Most poor grade patients who do well are able to follow commands within 5 days of aneurysm rupture whereas those who die may do so within the same time frame.73,74,77 In addition, patients who do poorly frequently develop intractable intracranial hypertension and progressive low density changes on follow-up CT scan.37,74 A suggested management algorithm for poor grade patients is illustrated in Fig. 362-4.
Endovascular occlusion of the acutely ruptured aneurysm is an attractive alternative to surgery for poor grade patients. However, the role of endovascular therapy in these patients has only been described in limited clinical series and very few poor grade patients were included in the ISAT trial.55,56,78–82 The few case series published suggest that endovascular therapy is a reasonable option in some poor grade patients, and that combined endovascular and open surgical therapies may be beneficial, particularly in the presence of ICH. For example, Suzuki and associates83 described 80 Hunt and Hess grade IV and 31 grade V patients, including 7 whose aneurysm was coiled and then had a craniotomy for hematoma evacuation. Overall mortality was 32.4% and a favorable outcome (mRS 1-2) was observed in 43.8% of grade IV and 12.9% of grade V patients.
A potential advantage of endovascular therapy for poor grade patients is that it may be physiologically less stressful because brain retraction and vessel dissection is not required. Consequently, endovascular therapy may be the preferable treatment for poor grade patients when extensive cerebral swelling is seen on CT scan or ICP is not controlled. In addition, endovascular therapy may be useful in elderly patients where long-term coil stability may be less relevant (Fig. 362-5). However, in the elderly, outcome may be less than expected after GDC treatment, in large part because there is 3 times greater risk of thrombo-embolic complications from atherosclerosis.84 Surgery, however, should be the primary treatment in young patients, when the ruptured aneurysm is associated with an ICH, the aneurysm is associated with mass effect, or the aneurysm, such as a wide-necked lesion, is not suitable for coil occlusion. There has been limited comparison of the use of endovascular techniques for poor grade patients to other management strategies. Inamasu and associates,82 using historical controls, observed no benefit to using endovascular techniques over a conservative policy; what determined outcome was not how the aneurysm was occluded, but rather in what condition the patient was and how the consequences of the SAH were managed. Groden and associates85 compared outcome in patients treated using surgical (n = 20), endovascular (n = 20), or by both modalities (n = 1); there was no difference in outcome.
Intracerebral Hemorrhage
ICH increases mortality after SAH.86–88 A single randomized study89 and several clinical series demonstrate a tendency for improved patient outcome when emergency ICH evacuation is performed, particularly when simultaneous aneurysm obliteration is achieved.75,90,91 Factors such as young age, better clinical grade, and small ICH volume (<25 mL) are associated with better outcome. However many comatose patients, including those with brainstem compression and large ICH can experience a favorable outcome if rapidly resuscitated and operated on within a few hours of aneurysm rupture.75 We obtain angiography on patients with suspected aneurysmal ICH provided they are neurologically stable. In the unstable patient, however, even single-vessel angiography may cause a life-threatening delay. Infusion CT scan, or CTA92–95 obtained immediately after the head CT scan, are useful in these patients; both techniques are rapid and can detect greater than 90% of aneurysms greater than 3 to 5 mm in diameter. In the neurologically unstable patient we then proceed to craniotomy, ICH evacuation, and aneurysm obliteration based on the CT angiogram alone (see Fig. 362-4). With this strategy we have found that 30% of moribund patients with clinical and CT evidence of brainstem compression after aneurysmal ICH are independent and living at home with only mild disability 6 months later.75 The role of endovascular aneurysm occlusion and then ICH evacuation83,96 and the role of decompressive craniectomy are attractive management strategies but their use is not yet well defined in SAH.
Acute Intraventricular Hemorrhage and Hydrocephalus
Acute hydrocephalus (ventricular enlargement within 72 hours) and IVH often are observed after aneurysm rupture, particularly in poor grade patients and those with thick subarachnoid blood on CT,97–100 although increased ICP also is seen in as many as 50% of good grade patients.25 External ventricular drainage (EVD) is recommended, particularly when the patient has depressed consciousness: 40% to 80% have some degree of improvement after the procedure.97,100 Several clinical series describe good results using EVD for hydrocephalus or IVH following aneurysm rupture provided early aneurysm occlusion is achieved.98,101 Attempts to remove casted intraventricular blood through craniotomy or the infusion of urokinase, however, do not appear to improve outcome.102,103 “Routine” EVD use in grade IV and V patients is advocated; the role in grade III patients is less certain.104 One recent study found a significant reduction in poor outcomes and clinical vasospasm using a lumbar drain placed intraoperatively and kept for 24 or more hours; however, this finding has not been replicated and the study has several methodological limitations.105 Clinical improvement within 24 hours of starting EVD suggests a favorable outcome; however, clinical improvement after ventricular drainage is not always associated with a favorable outcome.76,77,99,100 In addition, many patients who do not improve can undergo surgery with satisfactory results. Several authors have observed that EVD increases the risk of aneurysm rebleeding97,101,106,107 or impairs natural mechanisms that arrest aneurysm rupture.108 Ventricular drainage should therefore be performed carefully to prevent altering aneurysm transmural pressure that may precipitate rebleeding. Acute hydrocephalus is often associated with vasospasm, as well as intracranial hypertension and reduced cerebral blood flow (CBF);25,109–111 ventricular drainage should therefore be accompanied by early aneurysm occlusion to allow effective use of hyperdynamic therapy and angioplasty.
Chronic hydrocephalus is observed in about 25% who survive aneurysm rupture and its neurological and/or medical sequela.110 Half the patients with acute hydrocephalus eventually require a ventriculoperitoneal shunt. Factors associated with hydrocephalus include older age, increased ventricular size, and IVH at admission; poor clinical grade; preexisting hypertension; alcoholism; female sex; increased aneurysm size; pneumonia; and meningitis.110 The rate of shunt dependency is associated with a worse clinical grade, a higher Fisher computed tomographic grade, IVH, repeat SAH, and aneurysms arising at the anterior communicating artery. The need for a permanent shunt can be reduced by EVD, including long tunneled catheters, serial lumbar punctures, and perhaps by lamina terminalis fenestration at craniotomy.97,104,112 Though several series suggest that whether an aneurysm is occluded using surgery or endovascular techniques does not influence the subsequent risk for hydrocephalus,113–115116 a recent meta-analysis by de Oliveira and associates113 found significantly greater risk for shunt dependency after coiling than after clipping. Though coiling may not cause shunt-dependent hydrocephalus, it appears clipping may offer a reduced risk, possibly through clot evacuation. The implications of these studies are limited, however, because clot evacuation was not quantified in any of the studies and, at least in the study by de Oliveira and associates, IVH was significantly more common in the coiled group, which may represent a significant confounder.113
Ruptured Aneurysms and Early Vasospasm
Vasospasm is associated with poor outcome; between 10% and 15% of patients will have angiographic evidence of vasospasm within 48 hours of aneurysm rupture.117 What management is appropriate for these patients? Recent experimental and clinical observations suggest that surgical manipulation of blood vessels may not exacerbate the arterial narrowing seen after SAH.118–121 For example, in patients undergoing preoperative and postoperative angiography, surgical timing does not alter the incidence of angiographic vasospasm,119 or correlate with infarction, provided prophylactic hypervolemia is instituted early.121 Qureshi and associates117 in an analysis of 296 patients in the placebo arm of a multicenter trial found that early vasospasm is associated with subsequent poor outcome and an increased risk of symptomatic vasospasm. However, early surgery reduced the risk of poor outcome in these patients. We have found that prompt aneurysm obliteration, followed by immediate angioplasty for patients having symptomatic vasospasm and an unsecured aneurysm is feasible and offers a reasonable chance of neurologic recovery to these patients who might otherwise progress to cerebral infarction.122 The success of angioplasty is less dependent on angiographic success but rather on early intervention.123 In poor grade patients who cannot be reliably evaluated clinically, prophylactic angioplasty may be feasible in select patients with severe vasospasm.124 These patients may be selected for treatment based on cerebral blood flow studies or microdialysate analysis of extracellular metabolites for delayed ischemia.125
Surgical Complications After SAH
Surgical complications, such as intraoperative aneurysm rupture, major vessel occlusions, cerebral contusion, or ICH are associated with about 10% of the morbidity and mortality after SAH.39,45,65,126–132 Factors such as inexperience or poor surgical technique may play a role.127,131 However, several series suggest that surgical complications after SAH are primarily associated with aneurysm location, size, and morphology.61,64,128–130,132,133 In particular, large or giant aneurysms, aneurysms with atherosclerotic necks, or aneurysms located at the basilar bifurcation or anterior communicating artery are more frequently associated with surgical complications. The Cooperative Study and several studies comparing patient cohorts or historical controls suggest that although brain swelling is more common during early surgery, surgical timing is not associated with surgical complications.42,43,46,129 Similarly we found that the incidence of surgical complications is not influenced by clinical grade.39 However, it is not clear whether the impact of a surgical complication in a poor grade patient has greater adverse impact than the same complication in a good grade patient. Similarly, whether retraction of the swollen brain results in neuropsychological or cognitive deficits that may not occur in delayed surgery when the brain is less swollen, is not defined. However, recent data using neuropsychologic testing demonstrate no association between timing of surgery and neuropsychological outcomes.52,134–141
Where Should Patients with Intracranial Aneurysms Be Managed?
The association between surgical volume and outcome is well described for a variety of surgical procedures. In particular, policies that discourage coronary artery bypass surgery at low-volume hospitals are credited with a substantial decline in mortality associated with this procedure in New York and Canada.142 Similarly, the development of level I trauma centers is associated with better patient outcome and a reduction in preventable mortality.143–146 The management of aneurysmal SAH has several parallels with trauma and there is a growing body of evidence that patients with cerebral aneurysms should be managed in specialized centers by experienced practitioners.12,13,141,147–150 Several years ago, Solomon and associates150 and Taylor and associates,151 who incorporated all hospitals in a geographic region, observed that patient mortality, both for ruptured and unruptured aneurysms, was significantly lower in institutions that frequently performed aneurysm surgery. Both authors observed that teaching hospitals fared better than nonteaching hospitals. More recent studies based on administrative data have observed a significant difference in outcomes between high- and low-volume centers.12,13,149 For example, Berman and associates13 examined data from 1995 to 2000 at hospitals across New York and found among 5963 patients with an intracranial aneurysm who were treated with surgery or endovascular therapy that hospitals that performed more than 35 annual aneurysm procedures had lower death rates than low-volume hospitals. This effect was modest for ruptured aneurysms and greatest among unruptured aneurysms: for each additional 10 cases performed annually, the adverse outcome odds ratio (OR) was 0.85 for ruptured aneurysms and 0.89 for unruptured aneurysms. However, few patients (<5%) are transferred to high-volume centers.12 A cost benefit analysis performed on the same California dataset suggested that transfers to high-volume centers would result in significant improvements in quality-adjusted life years at an acceptable economic cost.148 In addition, the availability of both endovascular and surgical means of aneurysm occlusion within an institution appears to improve overall institutional outcome, though in at least one large study this effect was smaller than that of procedural volume.13,152
There are limitations to these various studies which are largely from secondary sources or administrative data. Nevertheless, the findings are compelling and, particularly in light of the findings from the International Subarachnoid Aneurysm Trial,55,56 suggest that all SAH patients should be treated in centers with a particular interest in cerebrovascular disorders and neurointensive care and where both endovascular and neurosurgical techniques are available. In addition there should be a dedicated neurointensive care unit where patients are cared for in a team-based comanagement approach.153 However, because there are significant risks of transferring critically ill patients between centers, the true improvement in outcome is difficult to estimate and further study is required.154 There will still be patients whose only chance of survival will be immediate attention at local facilities (e.g., a patient with a large temporal lobe clot after an SAH from a ruptured aneurysm that evolves into a herniation syndrome). The role of tranexamic acid, a short-acting antifibrinolytic agent, to reduce the incidence of early rebleeding during transfer requires further study.28
Special Circumstances
Age
Many studies suggest advanced age is associated with poor outcome after SAH. However, other studies demonstrate that young and old people in the same clinical condition experience similar outcomes.52,135–141 There are several important caveats when examining the association between age and outcome: (1) older patients are frequently excluded from active treatment; this influences outcome; (2) older patients are more often in poor clinical grade, which has a greater impact on outcome than age;139,155,156 and (3) other variables such as hypertension or atherosclerosis are common in elderly patients; these factors may have an independent adverse effect on outcome or in multivariate analysis replace the effect of age.45,74,138,157 Although no randomized trial has specifically addressed the role of active treatment in elderly patients, several lines of evidence suggest that surgically treated patients do better than conservatively treated patients after aneurysm rupture.140,158 In ISAT, subgroup analysis of 278 good grade SAH patients with small anterior circulation aneurysms found that between 42% and 86% of patients had a 1-year favorable outcome that depended in part on aneurysm location and treatment type. The data also suggests that endovascular techniques should probably be the favored treatment for ruptured internal carotid and posterior communicating artery aneurysms, whereas elderly patients with ruptured middle cerebral artery aneurysms benefit from surgery.159 Consequently, we believe that withholding treatment on the grounds of advanced age may not always be justified. Instead, the decision to treat an elderly patient after a SAH should be considered in light of the natural history of the disease and the patient’s overall physiologic condition and associated risk factors. Atherosclerosis in the aneurysm and associated vessels frequently increases the technical risk of aneurysm occlusion in the elderly. Consequently these patients may be best treated at specialized centers.160
Although good results among patients older than 60 years old undergoing surgical repair of unruptured aneurysms are reported, advanced age is a risk factor.7,10,19,160 This increased risk is due in part to more medical complications among older patients. In addition, older patients are more likely to have atherosclerotic or calcified aneurysms or adjacent vessels both of which increase surgical risk.39 Very old patients may not benefit from unruptured aneurysm treatment. Decision analysis studies suggest that surgical treatment of unruptured aneurysms is beneficial provided that the patient’s life expectancy is greater than 13 years.161,162 Quality of life studies suggest that the quality of life is lengthened by 4 years in 40-year-old patients, 2.4 years in 50-year-old patients, 1.3 years in 60-year-old patients, and by 0.6 years in 70-year-old patients.163 These various studies make certain assumptions about surgical risk and risk of aneurysm rupture but suggest overall that surgery may be appropriate in a 64-year-old man or a 68-year-old woman.161 Patients older than 70 years old, particularly those with small asymptomatic aneurysms or giant posterior circulation aneurysms associated with few symptoms, should probably be followed with serial MRI, MRA, and CT angiography. In these patients, treatment probably offers little over the natural history of these lesions and should only be considered if symptoms progress or aneurysm growth is documented.
Pregnancy
The management of intracranial aneurysms during pregnancy is complicated by the physiologic changes that accompany pregnancy and by potential risks to the fetus when investigating and treating the mother. Intracranial aneurysms are not commonly discovered during pregnancy, in part because the risk of hemorrhage is greatest during the late third trimester and delivery.164 Investigation of the pregnant woman with an aneurysm is similar to that for a nonpregnant female. However, the abdomen should be shielded with lead to reduce the risk of ionizing radiation to the fetus. In general, pregnant patients with ruptured aneurysms are treated the same as nonpregnant women with certain caveats. For example, during surgery temporary clips rather than hypotension are preferred to reduce the risk of fetal hypoperfusion. Similarly care should be taken when mannitol is administered because its use can lead to maternal hypoperfusion and subsequent uterine hypoperfusion, or fetal hyperosmolality. Hyperventilation can cause acid-base shifts and decreased oxygen delivery to the fetus. Once the aneurysm is occluded, pregnancy is allowed to proceed to term. If the patient is in good neurological condition, she should be able to deliver vaginally. When labor begins or is expected to begin immediately after aneurysm occlusion, then cesarean section is preferred. If aneurysm rupture occurs during labor, simultaneous craniotomy and cesarean section may be necessary; the cesarean section should be performed first so that the fetus is not exposed to prolonged anesthesia.165 Surgery should be deferred until the second trimester when an unruptured aneurysm is discovered during the first trimester, in part, to reduce potential drug teratogenic effects during treatment. Other medications such as anticonvulsants or calcium channel blockers should be avoided or used cautiously. A C-section may be preferable when an unruptured aneurysm is discovered late in pregnancy, particularly if the aneurysm becomes symptomatic or has enlarged. A vaginal delivery, using epidural anesthesia to shorten the second stage of labor, is acceptable in asymptomatic patients with an unruptured aneurysm. When needed and when appropriately performed, surgery for both the mother and fetus appears to be safe.166
Pediatric Aneurysms
Aneurysms in children are rare. In contrast to adults, posterior circulation, dissecting, giant, infectious, or traumatic aneurysms are more frequent.167–169 In small children, the operating room should be warmed and care taken to prevent intraoperative rupture because the total blood volume is relatively small.
Mycotic Aneurysms
Mycotic or infective aneurysms comprise between 2% and 6% of intracranial aneurysms in adults. These lesions often are associated with infective endocarditis or immunologic compromise.170 Fungal infections, particularly aspergillosis and candida, may be associated with proximal aneurysms. Aneurysms associated with bacterial infections, most commonly streptococci and Staphylococcus aureus, usually are located on distal middle cerebral artery (MCA) branches. All infective aneurysms require 4 to 6 weeks of culture-directed antimicrobial therapy whether the aneurysm is occluded or not. Antimicrobial therapy alone may be used to treat some infective aneurysms; between 30% and 50% resolve or decrease in size. The lesions require close follow-up with serial angiography and blood cultures. When aneurysm surgery is required, any hemodynamically significant cardiac lesion should be corrected before aneurysm obliteration. However, when the aneurysm is associated with an abscess or a hematoma, aneurysm surgery may be necessary first. During surgery the surgeon must be prepared for aneurysm excision and parent vessel anastomosis, bypass, or a ligation procedure. A short course of preoperative antibiotics may make the aneurysm and parent vessels less friable. The role of endovascular techniques to occlude infective aneurysms is not defined, in part because of concerns about placing a foreign body in an infected friable sac.
Traumatic Aneurysms
Less than 1% of all intracranial aneurysms are traumatic. Traumatic aneurysms usually are associated with penetrating head injury or contiguous skull fracture and are most frequent in the MCA region after low-velocity shrapnel injuries or stab wounds. However, basal skull fractures may be associated with basal traumatic aneurysms.171,172 The majority of traumatic aneurysms occur within 2 to 3 weeks of the original injury and are false aneurysms.170 A definable neck is rare and the lesions are prone to intraoperative rupture. Consequently the surgeon should be prepared to trap or excise the lesion. Encircling clips may also be useful. The surgical approach therefore must provide proximal and distal control and access to the superficial temporal artery or saphenous vein for a bypass if required. Endovascular techniques are feasible in some traumatic aneurysms173 and can effectively temporize a patient while the acute sequelae of the head injury resolve.174
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree