Chapter 13 Surgical Management of Brain Stem Tumors in Adults
Brain stem tumors account for 1.5% to 2.5% of all intracranial tumors in adults while comprising 10% to 20% of all pediatric tumors.1–4 These tumors are less common in adults and, therefore, more clinical studies have been conducted in children with brain stem tumors, specifically brain stem gliomas, since they are more common in this patient population.3–10 Adults with brain stem gliomas have a median survival of 5 to 7 years which is longer than that of children.2–5 Historically, brain stem tumors were treated as a homogenous group of lesions with radiotherapy, which usually proceeded without histopathologic confirmation. Surgery for these tumors was rare and typically limited to biopsy, extirpation of cystic lesions, and the placement of shunts for obstructive hydrocephalus. However, Pool documented one of the first surgical resections of a brain stem tumor located in the area of the cerebral aqueduct in 1968.11 This reported case led to the advent of further investigation and development of surgical techniques to treat lesions located in the brain stem.
Imaging and Classification
Prior to the advent of MRI, CT was utilized to assess the pathology of brain stem lesions and biopsy was often needed to guide management. MRI has become the primary diagnostic modality for patients with brain stem tumors because it has advanced the diagnosis and categorization of these lesions by providing superior anatomic detail of the brain stem and posterior fossa.12–16 T1- and T2-weighted MR imaging have provided the ability to differentiate the tissue characteristics of tumors in many cases. In adults, the differential diagnosis for brain stem lesions may be broad and may include such pathologic entities like metastatic tumors, demyelinating processes, infectious processes, granulomas, cavernous malformations, hemangioblastomas, or hematomas that should be differentiated from gliomas.
Based upon MRI and CT data, classification schemes have been developed for grouping brain stem tumors according to growth patterns and the feasibility of surgical resection (Table 13-1).13,14,17–21 The earliest classification schemes were based on CT images and surgical observation.17,19,21 Later schemes relied on MR imaging, in which better neuroanatomic details were made available.13,14,18 The most recently developed classification system relied on both CT and MRI.20 All of these schemes classify the tumor into either a focal or diffuse growth pattern, while the more complex classifications further subdivide the tumors into location within the brain stem, presence or absence of an exophytic component, and the presence of hydrocephalus or hemorrhage. The more complex schemes were developed in an attempt to predict tumor behavior and guide operative versus nonoperative management. In general, tumors with a focal growth pattern have been considered amenable to surgical extirpation in contrast to those with a diffuse growth pattern.
Table 13-1 Classification Schemes for Brain Stem Tumors
| Author | Method Used to Create System | Classification System |
|---|---|---|
| Epstein21 | CT | Intrinsic |
| Diffuse | ||
| Focal | ||
| Cervicomedullary | ||
| Exophytic | ||
| Anterolateral into cerebellopontine angle | ||
| Posterolateral and into brachium pontis | ||
| Disseminated | ||
| Positive cytology | ||
| Positive myelography | ||
| Epstein and McCleary19 | CT, MRI, and surgical observation | Diffuse |
| Focal | ||
| Cervicomedullary | ||
| Stroink et al.17 | CT | Group I—dorsal exophytic glioma |
| Group IIa—intrinsic brainstem tumors | ||
| Hypodense, no enhancement | ||
| Group IIb—intrinsic brainstem tumors | ||
| Hyperdense, contrast enhancing exophytic | ||
| Group III—focal cystic tumor with contrast enhancement | ||
| Group IV—focal intrinsic isodense contrast enhancement | ||
| Barkovich et al.14 | MRI | Location (midbrain, pons, medulla) |
| Focality (diffuse or focal) | ||
| Direction and extent of tumor growth | ||
| Degree of brainstem enlargement | ||
| Exophytic growth | ||
| Hemorrhage or necrosis | ||
| Evidence of hydrocephalus | ||
| Albright18 | MRI | Focal (midbrain, pons, medulla) |
| Diffuse | ||
| Fischbein et al.13 | MRI | MidbrainDiffuse |
| Focal | ||
| Tectal | ||
| Pons | ||
| Diffuse | ||
| Focal | ||
| Medulla | ||
| Diffuse | ||
| Focal | ||
| Dorsal exophytic | ||
| Choux et al.20 | CT and MRI | Type I—diffuse |
| Type II—intrinsic, focal | ||
| Type III—exophytic, focal | ||
| Type IV—cervicomedullary |
Focal Tumors
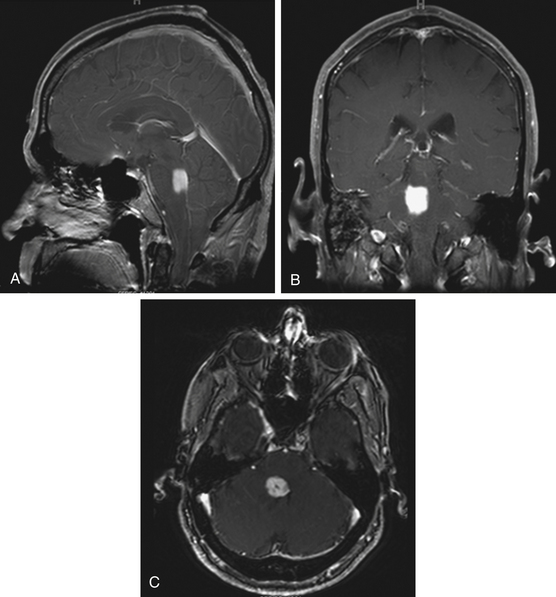
Focal tumors are intrinsic to the brain stem and may be cystic or solid (Fig. 13-1). They are typically not associated with edema and contrast enhancement may be variable. A majority of these tumors are low-grade gliomas, but some malignant tumors, such as a World Health Organization (WHO) grade-IV astrocytoma, may imitate a focal one, known as a pseudofocal tumor. In pseudofocal tumors, MRI and CT imaging demonstrate focal enhancement after the administration of contrast with normal signal characteristics from the peritumoral area that imitates the focal lesion.
Furthermore, the majority of cervicomedullary gliomas are low-grade, noninfiltrative tumors, and their growth is usually confined rostrally by the white matter of the corticospinal tract and medial lemniscus.22
Exophytic Tumors
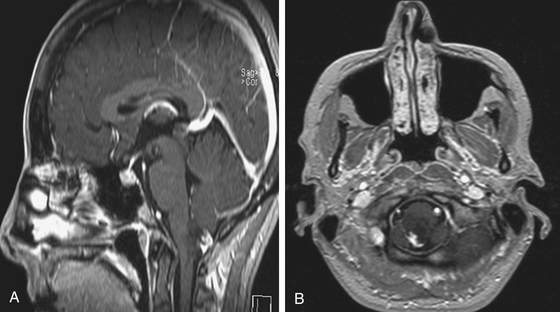
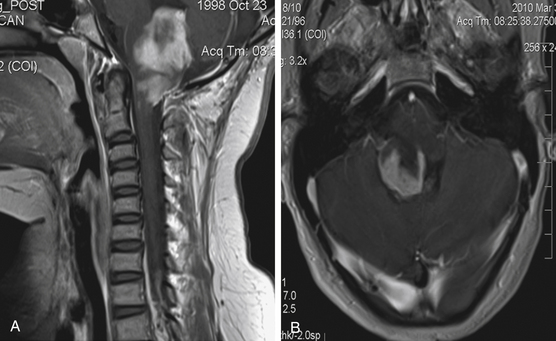
Focal brain stem tumors with an exophytically growing component are usually low-grade and well circumscribed (Fig. 13-2). They are typically dorsally exophytic tumors growing into the fourth ventricle or cervicomedullary tumors with exophytic growth into the cisterna magna and fourth ventricle (Fig. 13-3). It should be mentioned that, in addition to focal tumors, some diffuse tumors may cause bulging into the fourth ventricle, cerebellopontine angle, and prepontine and other cisterns.
Diffuse Tumors
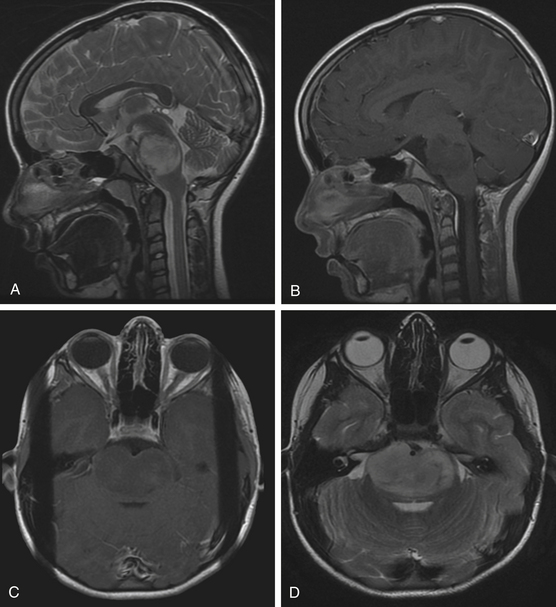
Diffuse brain stem tumors appear hypo- to iso-intense and hyper-intense on T1-weighted and T2-weighted MR images, respectively (Fig. 13-4). There is variable enhancement with contrast administration, and contrast enhancement in these tumors may be indicative of malignant degeneration. Moreover, the tumor boundaries are not able to be delineated on MRI, and the brain stem is typically enlarged and deformed.
Additional Imaging Modalities
Imaging of the central nervous system has evolved with the continual development of more sophisticated techniques. Positron emission tomography (PET) has been used in an attempt to differentiate low-grade from high-grade brain stem gliomas, particularly in the pediatric population.23–26 Although the MR imaging characteristics of gliomas may be highly diagnostic in the pediatric population allowing for the potential use of PET imaging to attempt to determine the degree of malignancy, the heterogeneity of lesions in the adult population may not allow for the procurement of useful data until studies are conducted correlating preoperative PET imaging with histologic diagnosis. One study utilized PET imaging in two adults with dorsal midbrain lesions and obstructive hydrocephalus.27 The lesions were hyperintense on T2-weighted images and partial contrast enhancement was observed in one patient. The patients underwent preoperative PET studies followed by an endoscopic third ventriculostomy combined with endoscopic biopsy sampling of the lesions. Glial proliferation, which contained the partial enhancement with contrast, and a possible low-grade glioma were diagnosed in the two patients, respectively. Results of the PET imaging in both patients suggested that the lesions had nontumorous characteristics and portended a good prognosis. With larger-scale studies, PET imaging may someday prove to be more informative and predictive of the biological behavior of dorsal midbrain lesions than a biopsy.
Magnetic resonance spectroscopy (MRS) is a noninvasive imaging modality used for tissue characterization and complements data obtained from MRI. The concentrations of creatine, phosphocreatine, choline, N-acetylaspartate, lactate, and lipids are analyzed in an effort to differentiate various pathologic processes. It has been used to distinguish between normal and abnormal tissues and grading of brain tumors. Inflammation, infectious processes, and tumors may potentially be distinguished with MRS.28 Studies have been conducted with MRS to investigate brain stem lesions with histologic correlation in some cases.28–31 MRS can provide additional information on brain stem lesions, but further studies are needed with histologic confirmation given the broad differential diagnosis of brain stem lesions in adults.
Diffusion tensor imaging (DTI) is an imaging modality that demonstrates white matter tracts. The relationship of sensory and motor tracts to brain stem tumors has been investigated in pediatric patients.32,33 With further studies and evolution of this technique for use in the brain stem, DTI may play a role in assisting the treatment planning for brain stem lesions in adults.
Clinical Manifestations
The clinical presentation of brain stem tumors varies and has been correlated with the growth pattern, location, and degree of malignancy. Focal tumors tend to have a slow progression to neurologic signs in contrast to diffuse malignant tumors, which have a fast progression to neurologic signs. The manifestations include headaches, nausea, vomiting, diplopia, weakness, ataxia, numbness, cranial neuropathies, and/or vertigo. Pontine and cervicomedullary tumors typically present with cranial neuropathies and long tract signs. Midbrain tumors can present with obstructive hydrocephalus, oculomotor deficit, hemiparesis, and ataxia, while dorsally exophytic tumors typically present with signs of obstructive hydrocephalus.
Stereotactic Biopsy
Since the late 1970s, stereotactic biopsies of brain stem lesions have been performed and account for 5% to 12% of all brain biopsies.34–56 This procedure was not used widely in the pediatric population because it was reported that characteristic MRI features were sufficient to diagnose diffuse brain stem gliomas without the need for a biopsy.14,57–59 Biopsies of brain stem lesions are not as common as other brain biopsies because of the risk involved in obtaining tissue. However, studies have shown that stereotactic brain stem biopsies can be routinely performed in a safe and effective manner.34,40,42–46,51–56 In some published series, the reported rate of complications has ranged between 2.5% and 7.7%.54,56,60
In adults with contrast-enhancing brain stem lesions, other pathologic processes must be considered in the differential diagnosis because studies have demonstrated preoperative radiographic diagnoses to be incorrect in 10% to 25% of cases in patients over 20 years of age presenting with a contrast-enhancing lesion in the brain stem.60,61 In addition to malignant glioma, the differential diagnosis may include infectious processes, such as abscess, tuberculomas, and toxoplasmosis, demyelinating disease, sarcoidosis, progressive multifocal leukoencephalopathy, metastasis, lymphoma, and vascular processes, such as vasculitis and infarction (Figs. 13-5, 13-6, and 13-7).2 This heterogeneity of brain stem lesions in adults makes it difficult to make a diagnosis on the basis of imaging alone. Therefore, image-guided stereotactic biopsies are indicated in many adult brain stem lesions that enhance with contrast and generally in cases in which the diagnosis of the lesion is in doubt to determine the histology of the abnormal process.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree