Chapter 82 Surgical Management of Infratentorial Arteriovenous Malformations
Introduction
Brain stem and cerebellar arteriovenous malformations (AVMs) are rare vascular lesions that represent only a small fraction of all intracranial AVMs. Whereas brain stem AVMs are less likely to be appropriate for surgery, given their involvement of vital neural structures,1 cerebellar AVMs are often treated surgically.2–6 Olivecrona in 1932 performed the first successful resection of an infratentorial (cerebellar) AVM.6,7 Current treatment algorithms for brain stem and cerebellar AVMs involve a multidisciplinary approach, where surgical resection, stereotactic radiosurgery (SRS), and endovascular embolization are used alone or in combination.8–12
Anatomy and Classification
Infratentorial AVMs consist of lesions located in the brain stem and the cerebellum. Given their location in the posterior fossa, AVMs in these locations are usually discussed together, despite their marked differences in clinical presentation, pathophysiology, and treatment options.13 In this chapter we discuss them separately.
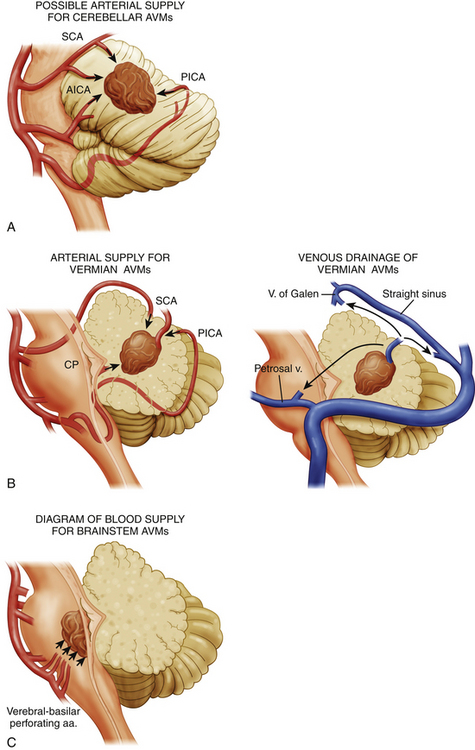
Brain stem AVMs encompass lesions in the midbrain, pons, and medulla. Angiography demonstrates the main arterial territories supplying the AVMs and facilitates their classification into three broad categories: (1) midbrain AVMs, supplied primarily by the superior cerebellar artery (SCA); (2) pontine AVMs, supplied primarily by the anterior inferior cerebellar artery (AICA); and (3) medullary AVMs, supplied primarily by the posterior inferior cerebellar artery (PICA) (Fig. 82-1).
Brain stem AVMs can then be subclassified according to the depth of their location into either superficial pial or parenchymal lesions. Pial brain stem AVMs are typically found in the anterolateral pons and are typically supplied by branches of an enlarged AICA, with venous drainage into the pontine-Galenic and petrosal systems,14 the mesencephalic tectum, or the floor of the fourth ventricle. Parenchymal brain stem AVMs have variable locations within the brain stem and typically extend into the cerebellar peduncles. The most important difference between these two groups, however, lies on their potential resectability. Whereas superficial pial AVMs may be resectable—given early visualization and control of their superficial blood supply, as well as limited manipulation of critical structures—parenchymal AVMs have an arterial supply primarily consisting of perforating arteries, and they often require an approach through critical nuclei and fiber tracts.14,15
Cerebellar AVMs can be anatomically classified into hemispheric, vermian, and tonsillar lesions. Each of these locations has a characteristic arterial supply and a unique relationship to the fourth ventricle. Cerebellar hemispheric AVMs are typically supplied by SCA, AICA, and PICA branches and rarely involve the fourth ventricular ependyma. Vermian AVMs are most often supplied by the SCA and PICA and can extend into the fourth ventricular roof. Tonsilar AVMs are supplied by PICA branches and often project dorsally, away from the fourth ventricle (Fig. 82-1).
Although the Spetzler and Martin AVM classification16 has been widely used and validated in supratentorial AVMs, this grading system, which takes into consideration size of the nidus, pattern of venous drainage, and location in eloquent areas, has been partially validated in infratentorial AVMs in only a few studies.2,8,10
Epidemiology
The annual incidence of brain AVMs has been estimated to be approximately 0.82 to 1.8 cases per 100,000 individuals.17–19 The prevalence of AVMs is more difficult to ascertain, given that many are asymptomatic. In a total of 17 studies discussing the epidemiology of AVMs,18–34 11 were population studies18–22,24,25,29,32–34 and 6 were autopsy studies.23,26–28,30,31 These studies were conducted in centers across the United States,19,23,24,30,32–34 Europe (Austria, Finland, Scotland, and Sweden),20,25–28 the Caribbean Islands,18 and Australia.21 In these studies, the prevalence of AVMs ranged from 5 to 613 cases per 100,000 individuals. We then calculate the mean prevalence of AVMs to be 0.3% based on 284 cases per 100,000 individuals, with a 95% confidence interval (CI) of 108 to 461 cases per 100,000 individuals.
Infratentorial AVMs represent at most 20% of all intracranial AVMs.9,14,35–37 However, because many of these malformations remain asymptomatic, a true estimate of the prevalence of posterior fossa AVMs has not been formulated. Cerebellar AVMs constitute 12% to 16% of all intracranial AVMs and are four times more frequent that brain stem AVMs, which represent only 3% to 4% of all intracranial AVMs.14,38
Although patients with all infratentorial AVMs present at a mean age of 42 years,39 those with brain stem AVMs present earlier, with their first symptoms occurring at a mean age of 32 years (range 9-65 years).9 Patients diagnosed later in life with infratentorial AVMs tend to present with a hemorrhagic event.39
Although gender predominance has not been demonstrated in infratentorial AVMs, some studies report a slight female predominance.9,12,39 Males with brain stem AVMs have a slightly higher risk of hemorrhage as opposed to females.39
Natural History
Infratentorial AVMs are rare entities that, in contrast to their supratentorial equivalents, present more commonly with hemorrhage (1.99 odds ratio, 1.07-3.69 95% CI).39 The size of the AVM is a controversial risk factor for the first bleeding episode, since different analyses have yielded contradictory results.40 Nevertheless, patients with small infratentorial AVMs may present more frequently with hemorrhage than those with large AVMs, given that small malformations otherwise remain asymptomatic.40 Each episode of hemorrhage is accompanied by a 30% morbidity and a 10% mortality. Morbidity due to bleeding of posterior fossa AVMs is high given their critical location. Patients with posterior fossa AVMs have a high mortality associated with the first hemorrhagic episode (66.7%). In some studies, however, authors have found only a 27% mortality from the initial bleeding episode.36 Subsequent hemorrhagic episodes often result in devastating neurologic deficits and have a mortality of 35.7%.8,13 Risk factors for hemorrhage identified for all brain AVMs—but not necessarily for infratentorial AVMs—include young age, large size of the malformation, deep venous drainage, associated aneurysms, and infratentorial location.1,40,41
The annual risk of hemorrhage of infratentorial AVMs appears to be higher than that of all AVMs and ranges from 8.4% to 13%, but it is higher in patients with a prior hemorrhage.2,8,39 After the first hemorrhagic episode and during the first 5 years after the same, infratentorial AVMs tend to hemorrhage repeatedly at a rate of 11.6% annually.40 After this 5-year period, infratentorial AVMs carry an inherent risk of subsequent hemorrhage of 7.5% at 5 years and of 5% at 10 years.42,43 Prospective studies have found that infratentorial AVMs have a higher annual rupture rate after the initial hemorrhage as compared to that of supratentorial AVMs.40,41,44 Other studies have found an overall annual risk for subsequent hemorrhage of 8.4%, which increases to 9.4% in patients who initially presented with a hemorrhage.8 The presence of associated aneurysms is another risk factor for poor outcome and subsequent hemorrhagic episodes. Hemodynamic factors that increase pressure gradients in the AVM, such as deep venous drainage, may also predispose patients to repeated hemorrhages.35 Differences regarding the site of venous drainage or the location and degree of venous stenosis also affect bleeding rates of AVMs.45,46
Clinical Presentation
Patients with infratentorial AVMs can present with headaches, neurologic deficits, or pain.38 Hemorrhage appears to be the most frequent clinical presentation and is more common in patients with small infratentorial AVMs (less than 2 cm in size).47 Hemorrhagic episodes can be related to associated aneurysms on the feeding arteries of these lesions.1 In patients with fourth ventricular hemorrhages, initial symptoms include those of obstructive hydrocephalus.
Less often, patients may present with progressive neurologic deficits but without hemorrhage. Under these circumstances, patients can present with a diverse array of symptoms, including headache, cranial nerve deficits, ataxia, dizziness, or hemiparesis. Although rare, infratentorial AVMs can cause mass effect and compression of important structures, especially in the confined space of the posterior fossa. While cerebellar compression may result in ataxia, dysmetria, and nystagmus, compression of the brain stem can affect different cranial nerve nuclei or traversing nerve fibers and may even present as trigeminal or glossopharyngeal neuralgia or as hemifacial spasm. Compression of the motor and sensory tracts may lead to hemiparesis and hemihypoalgesia.12,15,48
Preoperative Considerations
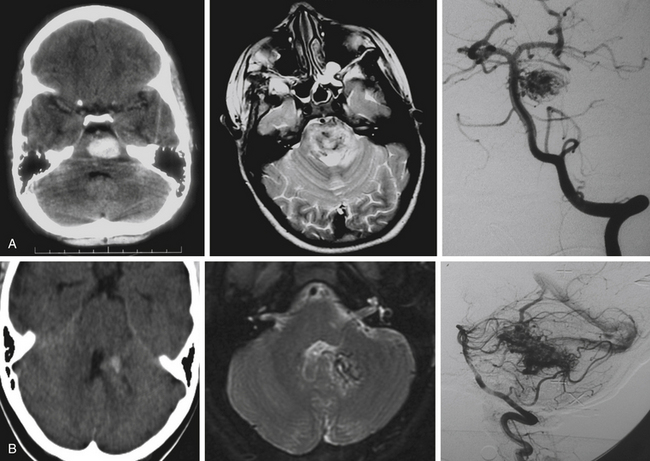
The three imaging studies needed to fully evaluate posterior fossa AVMs and plan their treatment are computed tomography (CT), magnetic resonance imaging (MRI), and digital subtraction angiography (DSA) (Fig. 82-3). CT scan best detects blood intraparenchymally, intraventricularly, or in the subarachnoid space, and is useful in identifying calcifications within the AVM. MRI reveals in detail the anatomic location of the AVM and its relationship to the surrounding structures. DSA provides critical information about feeding arteries and draining veins, as well as the structure of the nidus (i.e., compact vs. diffuse). All three studies are essential in the evaluation of an AVM.
CT scans are most useful in the initial evaluation of AVMs and in emergency settings in which the patient can quickly deteriorate as a result of intraparenchymal or intraventricular hemorrhage with hydrocephalus. CT scans detect approximately 95% of AVMs when contrast is used and about 84% when contrast is not used.48 Hyperdense lesions are seen either intraparenchymally (i.e., after hemorrhage in the subarachnoid space), or intraventricularly.48 Computed tomography angiography (CTA) may be useful in the initial evaluation of arterial supply and venous drainage of these lesions, and it may show enlarged arteries and draining veins, but CTA is not a substitute for DSA.49
MRI shows the anatomic relationship of the AVM to surrounding neural and vascular structures, either localizes the lesion entirely within the brain stem parenchyma or shows its pial representation, and can assist in determining previous bleeding episodes through hemosiderin-based sequences.50 In T2 sequences, flow voids are evident and a hyperintense signal may be noted surrounding the lesion. Fluid-attenuated inversion recovery images show a hyperintense signal surrounding the AVM due to gliosis. Magnetic resonance angiography and venography can be misleading in AVMs because of the intermediate flow characteristics of the AVM vasculature (which is partially arterial and partially venous) and because turbulence and artifact from bony obstructions often encountered in the posterior fossa.49
The critical study for the diagnosis and evaluation of infratentorial AVMs is DSA with selective catheterization, which defines the arterial supply of the AVM, its venous drainage, and the size of the nidus.49 In approximately 10% of posterior fossa AVMs, DSA shows a significant dural arterial component that originates from external carotid artery branches.51 Also of critical importance are the presence of associated aneurysms and the visualization of en passage feeding arteries. These arteries do not connect directly to the malformation but go through and give branches to it, and they must be identified early on, since these vessels also supply critical structures in the brain stem.3
In preparation for surgery, size reduction of infratentorial AVMs may be possible using either endovascular embolization or SRS. The vascular supply to brain stem AVMs, however, makes endovascular therapies dangerous—particularly in parenchymal brain stem lesions, as perfusion through perforating arteries is frequently encountered and manipulation with a microcatheter and displacement of embolization materials can result in ischemia and stroke. The mortality and morbidity associated with embolization of brain stem AVMs can be high. In a series of cerebral AVMs treated with embolization, the authors found a mortality rate of 1.3% and a rate of moderate and severe complications of approximately 20%.52
SRS is often used in surgically inaccessible brain stem AVMs and has been shown to reduce the risk of hemorrhage.10 In addition, SRS can be useful as a preoperative adjunct prior to microsurgical resection and in some series has minimized the need for embolization, decreased operative time, shortened hospital stay, and lowered morbidity.53