Chapter 69 Surgical Management of Intracerebral Hemorrhage
Epidemiology
Intracerebral hemorrhage (ICH), or hemorrhage within the brain parenchyma, occurs with an incidence estimated to range from 15 to 35 cases per 100,000 people per year. The incidence is up to twice that of subarachnoid hemorrhage by some estimates. Each year, approximately 37,000 to 52,000 people in the United States have an ICH. The rate is expected to double during the next 50 years as a result of the increasing age of the population and changes in racial demographics. A 1993 report found that only 38% of patients affected with ICH survive the first year,1 while a 2009 report found improvement up to 51% for 3-year survival in ICH patients.2
Six risk factors for ICH have been identified—age, male sex, race, hypertension, high alcohol intake, and low serum cholesterol. Regarding other possible risks, current or past smoking and diabetes mellitus are weak risk factors, if at all.3 The incidence of ICH increases significantly after age 55 and doubles with each decade of age until the age of 80, at which point the incidence increases 25-fold each decade.4 ICH is more common in men than women. ICH also affects blacks and Japanese more than whites. During the 20-year period covered by the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study, the incidence of ICH among blacks was 50 per 100,000, a little over twice the incidence among whites.5 It has been hypothesized that hypertension and factors leading to limited access to health care result in the higher incidence of ICH within the African-American community. The higher incidence of ICH in Japan has been attributed to a higher incidence of hypertension in Japanese populations and diets leading to low serum cholesterol, another risk factor for ICH. The reversibility of the dietary factor may lead to reductions in ICH seen when Japanese people emigrate to the United States, while their persistent hypertension may explain why their rates never drop to the same level as whites even after they emigrate to the United States.
There have been 11 case-controlled studies on hypertension and risk of ICH, with all showing a positive association between hypertension and ICH. Hypertension is classified as high normal (systolic 130–139 or diastolic 85–89), stage I hypertension (systolic 140–159 or diastolic 90–99), stage II hypertension (systolic 160–179 or diastolic 100–109), or stage III hypertension (systolic > 180 or diastolic > 110). Suh et al. found a relative risk of 2.2 for high normal, 5.3 for stage I hypertension, 10.4 for stage II hypertension, and 33 for stage III hypertension.6 Iribarren et al. found for each one standard-deviation increase in systolic blood pressure (18 mm Hg in men; 19 mm Hg in women) a relative risk of 1.14 in men and 1.17 in women.7 Leppala et al. found a relative risk of 2.20 for systolic blood pressure 140 to 159 mm Hg and 3.78 for systolic blood pressure greater than 160 mm Hg compared with systolic blood pressure less than 139 mm Hg.8 The correlation between blood pressure and ICH also leads to diurnal and seasonal variations in the onset of ICH. In general, ICH onset is usually during activity and rarely during sleep, which may be related to elevated blood pressure or increased cerebral blood flow. One study covering a decade of ICH cases in a Japanese city found that men 69 years of age and younger had a bimodal distribution of ICH-onset time, with an initial peak between 8:00 a.m. and 10:00 a.m., and a second, lower peak between 6:00 and 8:00 p.m. Men 70 years of age or older and women of all ages exhibited only a single evening peak, between 6:00 and 10:00 p.m.9 Men exhibited peak ICH in winter and a trough in summer, while women had no seasonal patterns.9 The incidence of ICH correlates with the daily times of blood pressure peaks in the sexes, and the ability of the autonomic nervous system to raise blood pressure during the winter may particularly affect men because they tend to work outdoors more often.
Alcohol consumption is a risk factor in both the short term and long term. During the 24 hours preceding an ICH, moderate alcohol consumption (41 to 120 g of ethanol, where one standard drink averages 12 g of ethanol) causes a 4.6 relative risk of ICH, while heavy alcohol consumption (>120 g of ethanol) causes an 11.3 relative risk of ICH. During the week preceding ICH, low (1–150 g of ethanol), moderate (151–300 g of ethanol), and heavy (>300 g of ethanol) alcohol consumption carry relative risks of 2.0, 4.3, and 6.5, respectively.10 ICH in patients with high ethanol consumption tends to be lobar.11 Ethanol promotes ICH by impairing coagulation and by directly affecting the integrity of cerebral vessels.11
A counterintuitive finding has been the identification of low serum cholesterol as a risk factor for ICH. Iribarren et al. found that for each one standard deviation increase in serum cholesterol (1.45 mmol/L in men and 1.24 mmol/L in women), there was a relative risk reduction of 0.84 in men and 0.92 in women.7 One potential mechanism may be that patients with low serum cholesterol may exhibit reduced consumption of animal products, and such patients will have reduced concentrations of arachidonic acid in their cell membranes.12 Arachidonic acid is a vital structural component of the cell membranes of vascular endothelium and its metabolites are involved in regulation of vascular tone and repair of injured vascular endothelium.12 Defects in this pathway may increase the risk of ICH. However, hypercholesterolemia is a proven risk factor for morbidities such as myocardial infarction that are far more common than ICH and should therefore be avoided. Furthermore, patients taking cholesterol lowering statin drugs before experiencing an ICH have been shown to have lower hematoma volumes, although the difference has not been shown to impact clinical outcome after ICH.13
Etiology
In primary ICH, the hemorrhage arises from vessels damaged by chronic hypertension or amyloid angiopathy. Chronic hypertension causes degenerative changes in the walls of small penetrating arteries originating from the anterior, middle, or posterior cerebral arteries. These changes reduce vessel compliance and increase the likelihood of spontaneous rupture. Patients with chronic hypertension incur an annual risk of recurrent ICH of 2%, but this risk can be reduced by treatment of hypertension.14 In 1868, Charcot and Bouchard attributed ICH to rupture at points of dilation in the walls of small arterioles that they called microaneurysms.15 These microaneurysms were later found to be subadventitial hemorrhages or extravascular clots resulting from endothelial damage by the hematoma. Electron-microscopy studies have since suggested that most ICH occurs at or near the bifurcation of affected arteries, where prominent degeneration of the media presumably caused by chronic hypertension can be seen.
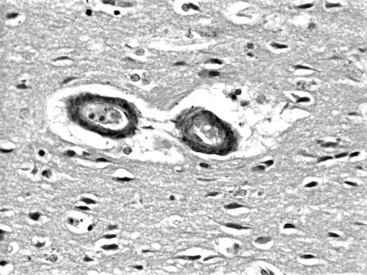
In amyloid angiopathy, β-amyloid protein, an acellular eosinophilic material, is deposited within the media of small- and medium-sized arteries in the cerebral cortex and leptomeninges, which causes primary ICH in the white matter of the cerebral lobes, particularly the parietal and occipital areas, in persons older than 70 years of age who exhibit no evidence of systemic amyloidosis. These patients face an annual risk of recurrent hemorrhage of 10.5%.16 Cerebral amyloid angiopathy is present in the brains of 50% of people over the age of 70; however, most do not experience ICH. Amyloid angiopathy may be associated with genetic factors including the apolipoprotein E allele and may be more prevalent in patients with Down’s syndrome. O’Donnell et al. reported that the presence of the ε2 or ε4 alleles of the apolipoprotein E gene was associated with a tripling of the risk of recurrent ICH among survivors of primary lobar ICH attributable to amyloid angiopathy.16 Among patients with lobar ICH, those with the apoE ε4 allele typically have their first ICH more than 5 years earlier than noncarriers (average age of 73 versus 79),17 and experience a statistically independent decrease in survival.18 Although they are distinct diseases, there is some overlap between amyloid angiopathy and Alzheimer’s disease, in that the amyloid in amyloid angiopathy is identical to that found in the senile plaques of Alzheimer’s disease and apolipoprotein-ε4 is associated with both the parenchymal plaque amyloid seen in Alzheimer’s disease and the deposits of β-amyloid protein in cerebral vessel walls seen in amyloid angiopathy. Cerebral amyloid angiopathy may increase the risk of ICH by potentiating plasminogen, a finding that may be of some relevance to patients receiving tissue plasminogen activator (t-PA) to treat myocardial infarcts or cerebrovascular accidents. Amyloid angiopathy can be diagnosed suggestively on the basis of radiologic findings such as hemosiderin deposits from small cortical and subcortical petechial hemorrhages on gradient-echo magnetic resonance imaging (MRI). Histologic findings include deposits of acellular eosinophilic material in the media of vessels in the hematoma or in noninvolved brain (Fig. 69-1). Perivascular microglia, thickened vessel walls, vessel dilatation, and microaneurysms are also seen in the vessels of patients with cerebral amyloid angiopathy. After staining with Congo red, the amyloid in the media of vessel walls exhibits apple-green birefringence under polarized light. A definitive diagnosis can be made on the basis of all three of the following findings: lobar, cortical, or corticosubcortical ICH; severe cerebral amyloid angiopathy on histopathologic exam; and absence of another diagnostic lesion. A probable diagnosis with supporting pathologic evidence occurs with all three of the following findings: lobar, cortical, or corticosubcortical ICH; some degree of vascular amyloid deposition on histopathologic exam; and absence of another diagnostic lesion. Probable amyloid angiopathy without pathologic evidence occurs with all 3 of the following findings: age over 60 years; a history of multiple hemorrhages in the lobar, cortical, or subcortical regions; and absence of another cause of hemorrhage. A diagnosis of possible amyloid angiopathy occurs with age over 60 combined with either a single lobar, cortical, or corticosubcortical hemorrhage without another cause or multiple hemorrhages with a possible but not a definitive cause.
Secondary ICH is far less common than primary ICH but because the etiologies of secondary ICH include tumors and vascular malformations that will need surgical intervention, or coagulopathies that need to be immediately corrected, attention must always be paid to secondary ICH as a possibility with any ICH. Tumors that produce ICH are usually malignant metastases. Hemorrhage is present in 3% to 14% of metastases and is most commonly seen in metastases from renal cell carcinoma, choriocarcinoma, melanoma, and renal cell carcinoma, with hemorrhage occurring in 70%, 50%, 40%, and 25% of the brain metastases from these respective primaries.19 However, bronchogenic carcinoma represents the most common source of hemorrhagic cerebral metastases because, although only 9% of metastatic bronchogenic carcinomas undergo hemorrhage, it is a much more common metastasis than the other four tumor types. When ICH appears on an initial CT scan, the presence of nonhemorrhagic necrotic or hypodense tissue and pronounced surrounding vasogenic edema are radiologic clues to the underlying neoplasm and warrant an MRI with gadolinium to look for tumor. Vascular malformations that can give rise to secondary ICH are usually arteriovenous malformations (AVMs), with 81% of hemorrhages from AVMs having a significant intraparenchymal component. Cavernous malformations also tend to cause hemorrhage with a significant intraparenchymal component, but only represent 10% of central nervous system vascular malformations. The diagnosis is strongly suggested by finding a mixed signal core indicative of old hemorrhage and a T2 dark rim on an MRI. ICH is unusual from aneurysmal rupture, which usually causes subarachnoid hemorrhage. Aneurysms that become adherent to the brain surface due to fibrosis from inflammation or previous hemorrhage can sometimes produce ICH rather than subarachnoid hemorrhage when they rupture. Oral anticoagulant therapy is a known source of secondary ICH. The relative risk of ICH during oral anticoagulant therapy increases more than 10-fold in patients over the age of 50.20 Bleeding is more protracted and hematomas larger in patients treated with anticoagulants than in those with spontaneous ICH.21 The management of these patients requires rapid reversal of their coagulopathy. Vitamin K provides long-term reversal and stabilization of the international normalized ratio (INR), while fresh frozen plasma (FFP) provides faster reversal, although one study showed that 24 hours after administering 1000 ml of FFP, patients on coumadin with ICH dropped their INR from 3.35 to 1.40.22 Slightly less than one third of these patients experienced radiographic hematoma enlargement within 24 hours of their initial CT scan,22 suggesting that the time it takes FFP to reverse a coagulopathy may be too slow, particularly in elderly patients who cannot tolerate rapid administration of volume. A suggested alternative is prothrombin complex concentrate (PCC), which can counteract the effects of warfarin as early as 10 minutes in much smaller volumes than FFP.22
Pathophysiology
The presence of hematoma initiates edema and neuronal damage in the surrounding parenchyma. Animal models of ICH have identified three phases of perihematoma edema—immediate (within 24 hours), intermediate (24 hours to 5 days), and late onset (from 5 days to several weeks after ICH). Immediate edema occurs within the first 24 hours and can often be seen at a histologic, but not radiographic level. This initial edema develops secondary to osmotically active plasma proteins accumulating in the extravascular space.23 The blood–brain barrier is intact at this point, so the proteins most likely arise from the hematoma. After the initial hemorrhage, the clotting cascade activates thrombin, which disrupts the blood–brain barrier and activates the complement cascade, leading to lysis of red blood cells and other bystander cells. Vasogenic edema and cytotoxic edema subsequently follow owing to the disruption of the blood–brain barrier, the failure of the sodium pump, and the death of neurons.24 This represents the intermediate edema seen at 24 hours to 5 days. This intermediate edema is noticeable radiographically and histologically. Red blood cell lysis releases hemoglobin and leads to formation of free radicals, which account for the late onset edema. The role of the coagulation cascade in intermediate perihematoma edema may explain why ICH related to thrombolysis or coagulopathy causes less perihematoma edema than spontaneous ICH.
Studies in animals and humans have refuted the notion that cerebral ischemia in areas of ICH occurs due to mechanical compression by the hematoma, and have suggested that secondary mediators may cause the delayed development of neuronal injury adjacent to a hematoma.23 It is currently thought that blood products mediate most secondary processes initiated after ICH.25 Recent evidence has suggested the presence of apoptosis or programmed cell death in neurons adjacent to ICH associated with nuclear factor-kB expression in neuronal nuclei.26 Other studies have suggested that heme derived from erythrocytes extravasated during ICH is degraded into bilirubin and bilirubin oxidation products, which activate microglia, which secrete cytokines that recruit leukocytes into the brain, which contribute to the injury process.27
Presentation by Location
Intracerebral hemorrhage into the cerebral white matter includes ICH into the occipital, temporal, frontal, and parietal lobes, including ICH arising from the cortex and subcortical white matter, as opposed to ICH of deep structures such as the basal ganglia, thalamus, and infratentorial structures. Frontal lobe ICH causes frontal headache with contralateral hemiparesis, usually in the arm with mild leg and facial weakness. Parietal lobe ICH causes contralateral hemisensory deficit with mild hemiparesis. Occipital lobe ICH causes ipsilateral eye pain and contralateral homonymous hemianopsia, with some sparing of the superior quadrant. Temporal lobe ICH can be asymptomatic on the nondominant side, but, on the dominant side, produces fluent dysphasia with poor auditory comprehension but relatively good repetition. Lobar ICH is more likely to be associated with structural abnormalities such as AVMs or tumors than deep hemorrhages. Lobar ICH is also more common in patients with alcohol consumption. In one study, significant independent risk factors for lobar ICH included the presence of an apolipoprotein E ε2 or ε4 allele, frequent alcohol use, prior stroke, and first-degree relative with ICH, while significant independent risk factors for nonlobar ICH were hypertension, prior stroke, and first-degree relative with ICH,28 suggesting different etiologies for lobar ICH than the other locations of ICH. Lobar ICH may also have a more benign outcome than basal ganglia and thalamic ICH.
In putaminal ICH, 62% of patients experience smooth gradual deterioration with only 30% exhibiting their maximal deficit at the onset. In some studies, the 30-day mortality rate has been 50%.29 The clinical presentation of putaminal hemorrhage may vary from relatively minor pure motor hemiparesis to profound weakness, sensory loss, eye deviation, hemianopsia, aphasia, and depressed level of consciousness. Headache is a presenting symptom in only 14% of putaminal ICHs. In putaminal ICH, intraventricular extension portends a poor prognosis, because the hematoma must be quite large to track through the internal capsule and reach the ventricle.
Patients with supratentorial ICH involving the putamen, caudate, or thalamus have contralateral sensory-motor deficits of varying severity due to involvement of the internal capsule. Higher-level cortical dysfunction, including neglect, gaze deviation, hemianopsia, and, for dominant hemisphere lesions, aphasia, can occur as a result of disruption of connecting fibers in the subcortical white matter and functional suppression of overlying cortex, known as diaschisis.30
Any deep, large hematoma can extend into the ventricles causing intraventricular hemorrhage (IVH). Common non-specific initial symptoms include headache and vomiting due to increased intracranial pressure and meningismus resulting from blood in the ventricles. As any hematoma becomes larger, patients will exhibit a decreased level of consciousness due to increased intracranial pressure and direct compression or distortion of the thalamic and brain-stem reticular activating system. Small, deep lesions can occasionally impair consciousness due to decreased central benzodiazepine receptor binding on cortical neurons.31
Cerebellar ICH can cause patients’ level of consciousness to progress from impaired to comatose due to direct compression of the brain stem, without any associated hemiparesis, unlike supratentorial ICH. Cerebellar ICH can present with the abrupt onset of vertigo, headache, vomiting, and inability to walk without any associated hemiparesis. Cranial nerve palsies are common, particularly an abducens palsy or a peripheral facial palsy. In one study, at least two of the three characteristic clinical signs—appendicular ataxia, ipsilateral gaze palsy, and peripheral facial palsy—were present in 73% of the cases of cerebellar ICH.32
Evaluation
Initial evaluation is typically through a CT scan, which is rapid and easily demonstrates ICH as high-density material within the brain parenchyma. Although mass effect on adjacent brain is common, the tendency for the hemorrhage to dissect through brain tissue often results in less mass effect than would be anticipated from the size of the clot. Clot volume can be estimated by computer programs that allow one to outline the hematoma on each slice and then model the hematoma in three dimensions and estimate a volume, or it can be approximated using the established practice of the modified ellipsoid volume, (A × B × C)/2, where A, B, and C are the diameters of the clot in each of three orthogonal dimensions, with one of the dimensions, C, being superoinferior such that C is equal to the number of slices with hematoma × slice thickness.33 Clot volume carries significant prognostic significance. One study demonstrated a much steeper dependence of mortality on clot volume for deep ICH than lobar ICH, consistent with the fact that deep areas of the brain are less able to accommodate large volumes. Hematomas were divided into small (≤30 cm3), medium (30–60 cm3), and large (≥60 cm3). The 30-day mortalities for small, medium, and large hematomas were 23%, 60%, and 71% for lobar ICH, compared to 7%, 64%, and 93% for deep ICH.34 The overall 30-day mortality was 39% for lobar ICH and 48% for deep ICH. Hematoma volume also correlates with risk of rehemorrhage, with one retrospective study showing that 39% of ICH patients who experienced rehemorrhage had initial clot volumes greater than or equal to 25 cm3, compared to only 23% of ICH patients who did not experience rehemorrhage.35
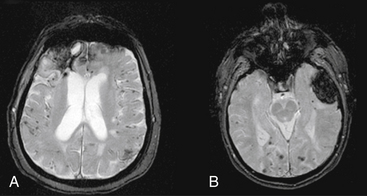
Magnetic resonance imaging is not the initial study of choice, as it is more time consuming, makes it difficult to access a patient who may acutely deteriorate, and does not show blood well within the first few hours. MRI may, however, may be useful later once a patient stabilizes to identify cerebral amyloid angiopathy, cavernous malformations, or underlying tumors. Gradient-echo MRI is the most useful modality for identifying ICH of various ages. Gradient-echo MRI increases the amount of signal dropout from deposits of iron representing residual blood products as a result of past hemorrhage. This increases the potential for detecting two findings that are typical of patients with cerebral amyloid angiopathy: (1) small prior punctuate petechial hemorrhages; and (2) previous lobar ICH, as manifested by a dark hemosiderin ring around an area of lobar encephalomalacia (Fig. 69-2). MRI has improved to the point that gradient echo can now detect ICH as early as 2.5 hours with 99.5% sensitivity.36 However, the specificity of MRI is limited in the hyperacute stage.37 Overall, MRI remains a secondary study compared to CT, and is most useful when a CT scan has findings listed above that suggest an underlying lesion such as a tumor or cavernous malformation. In these cases, MRI with gadolinium can be used to search for enhancing areas consistent with tumor; MR spectroscopy can be used to identify areas with high choline peaks, an inverted lactate peak, and absence of creatinine and N-acetyl-aspartate peaks; or T2 MRI can show a central mixed density core suggesting old hemorrhages surrounded by a hypodense rim.
Cerebral aniography is used to identify arteriovenous malformations (AVMs) and aneurysms in patients with ICH. In a prospective study in which 206 ICH cases were investigated with CT and angiography, anigoraphic yield was significantly higher in patients (1) at or below the age of 45, and (2) without preexisting hypertension.38 Analysis of angiographic yield of different sites of hemorrhage taken together with these two factors showed that (1) lobar ICH had a 10% angiographic yield in patients older than 45 with preexisting hypertension; (2) putaminal, thalamic, and posterior fossa hemorrhages in patients of all ages with preexisting hypertension had a 0% angiographic yield; (3) lobar ICH had a 65% angiographic yield in normotensive patients younger than 45; (4) putaminal, thalamic, and posterior fossa hemorrhages in normotensive patients younger than 45 had a 48% angiographic yield; and (5) putaminal, thalamic, and posterior fossa hemorrhages in normotensive patients older than 45 had a 7% angiographic yield. Isolated intraventricular hemorrhage (IVH) patients had 63% yield in the older and 67% yield in the younger groups. Taken together, these findings led the authors to recommend DSA in ICH patients except those older than 45 years who also have preexisting hypertension and thalamic, putaminal, or posterior fossa hemorrhages. More recently, the safer and more rapid technique of three-dimensional (3D) CTA has begun to replace DSA. CTA involves a head CT with thin 2.5-mm axial cuts occurring 5 seconds after administering a 45 mL contrast bolus at 7 cc/sec. Imaging software programs, which reformat the axial cuts and subtract out all but the contrast and adjacent brain tissue, are used to generate 3D images of the cerebral circulation. Preliminary studies have shown that CTA is 95% as sensitive and just as specific as DSA in the detection of cerebral aneurysms,39 a gap that is expected to close as CTA technology improves.
Acute Rehemorrhage
Although it was initially believed that ICH was largely a monophasic event that stopped quickly as a result of clotting and tamponade by the surrounding regions, a number of investigators have shown that rehemorrhage is common, with one study of 627 ICH patients showing a 14.0% rehemorrhage rate within 24 hours of admission.40 Brott et al. found that the hematoma expanded in 26% of ICH patients within 1 hour of the initial CT scan and in another 12% within 20 hours.41 Expansion has been attributed to continued bleeding from the primary source and to the mechanical disruption of surrounding vessels from compression by the hematoma. Acute hypertension after the initial ICH, a local coagulation deficit, or both may be associated with expansion of hematoma.35
Factors associated with rehemorrhage in the initial 24-hour period include: (1) a previous history of brain infarction, (2) liver disease, (3) uncontrolled diabetes, (4) systolic blood pressure on admission greater than 195, (5) a history of alcohol abuse, (6) coagulation abnormalities, (7) a hematoma larger than 25 cm3 on the initial CT scan, (8) irregular hematoma shape because irregularly shaped hematomas seem to indicate active bleeding from multiple sources, (9) a large peripheral white cell count, and (10) elevated body temperature on admission.40 CT angiography performed within 12 hours of the ICH revealing extravasation of contrast predicts rehemorrhage on a 24-hour post ICH CT scan with 60% specificity and 100% sensitivity.42
Systolic blood pressure control using an arterial line and intravenous drips of antihypertensives like nitroprusside remains the primary medical intervention designed to prevent rehemorrhage. Recommendations written by a panel of experts commissioned by the American Heart Association (AHA) in 1999 are to maintain the mean arterial blood pressure below 130 mm Hg (systolic BP < 180 and/or diastolic BP < 105) in patients with a history of chronic hypertension,43 but therapeutic trials are needed. In patients with elevated ICP documented by an ICP monitor, cerebral perfusion pressure should be kept above 70 mm Hg.