CHAPTER 370 Surgical Management of Middle Cerebral Artery Aneurysms
The middle cerebral artery (MCA) originates at the bifurcation of the internal carotid artery (ICA). It is the largest branch of the ICA and courses laterally within the sylvian cistern, providing branches along its course, ultimately ending as small cortical or deep lenticulostriate vessels. Approximately 14% to 20% of all intracranial aneurysms originate along the MCA,1–5 most often at the bifurcation of the first segment (M1) with the second segment (M2), and projecting laterally in the plane of the M1 segment.2 MCA aneurysms are somewhat unique for several reasons: they may grow to 20 mm or larger before detection, they are more likely to present with an intraparenchymal clot, and they are more likely to cause symptoms of mass effect than most other intracranial aneurysms.6 They are also, by virtue of their angioarchitecture, more challenging to treat with endovascular techniques than other aneurysms within the intracranial circulation.
Middle Cerebral Artery Anatomy
The sylvian fissure is the gateway to the mesial skull base and its related structures. The fissure is composed of a deep and superficial component. The deep component of the fissure is divided into the anterior (sphenoidal) compartment, which contains the M1 segment of the MCA, and the posterior (operculoinsular) compartment, which contains the M2 and M3 segments. This deep fissure is sometimes known as the sylvian cistern and is contiguous with the basilar cisterns. The superficial part of the sylvian fissure is composed of a stem, which extends from the anterior clinoid process in a medial to lateral direction between the frontal and temporal lobes, and several rami.7
Lying within the sylvian fissure, the MCA is the largest and most variable of the intracranial arteries. It is divided into four segments: M1 (sphenoidal), M2 (insular), M3 (opercular), and M4 (cortical). The M1 segment extends from the bifurcation of the ICA, coursing at the bottom of the sylvian fissure. The M1 segment can be of variable lengths but classically is longer than 15 mm. Short M1 segments have surgical implications because aneurysms on such vessels are, by definition, deeper within the fissure than ordinarily expected. The genu of the MCA is often at or just distal to the limen insulae (a small gyrus at the anteroinferior corner of the insula) and classically corresponds to the bifurcation (78%), trifurcation (12%), or multiple trunks that proceed distally (10%).8 M2 segmental arteries course within the fissure posterosuperiorly at variable depths and branch into M3 vessels at the periphery of the insulae but are variable, and end at the cortical surface where M4 vessels are identified.
The branches of the middle cerebral artery are important for surgical orientation, treatment paradigms and implications, and salvage techniques. The M1 has multiple lenticulostriate arteries that are divided into two groups. The medial lenticulostriate arteries enter the anterior perforating substance superiorly and supply the lentiform nucleus, the caudate, and the internal capsule. The lateral lenticulostriate arteries are more variable in their location, traverse the basal ganglia, and supply the caudate nucleus.9 The anterior temporal artery is a large cortical branch that arises from the M1 segment before the MCA bifurcation and is the first named branch of the M1, supplying the temporal tip. Anomalies of the MCA candelabra are not unusual and include duplications of the MCA, also known as accessory MCAs (1% to 3%),9 arising from the anterior cerebral artery (ACA) and paralleling the MCA (2.7%),10 and other anomalies have been reported and associated with an increased incidence of aneurysm formation.11,12
The venous anatomy of the sylvian fissure is much more variable. The preservation of these veins during opening of the sylvian fissure and aneurysm dissection is critical in preventing venous congestion or even venous infarction.13 We have found that with few exceptions, the sacrifice of any veins is unnecessary, and even frontobasal veins that arise from the temporal side of the fissure can be easily and safely accommodated.
Classification of Middle Cerebral Artery Aneurysms
Classification by Location
Aneurysms of the M1 segment are second in frequency to bifurcation aneurysms and are composed of lenticulostriate or anterior temporal artery saccular aneurysms. Proximal M1 segment lesions represent 2% to 12% of all MCA aneurysms.6,14,15 In patients with multiple intracranial aneurysms, the frequency of proximal MCA aneurysms tends to increase, and nearly three fourths of patients with multiple intracranial aneurysms harbor an MCA aneurysm.6 In fact, Rinne and colleagues examined 561 patients with MCA aneurysms and found that 39% of patients with MCA aneurysms had multiple intracranial aneurysms, significantly higher than the 20% classically quoted for other parts of the intracranial circulation.4,6,16 Additionally, these investigators found that the multiplicity of aneurysms increased the risk for poor outcome.6
Proximal MCA aneurysms can be further divided into superior wall type and inferior wall type, depending on the specific anatomic presentation.17 Superior wall–type M1 segment aneurysms arise at the origins of the lenticulostriate arteries and project into the frontal lobe posterosuperiorly.17 All efforts must be made to preserve such perforating vessels.18–20 Such aneurysms are also often small, making them hazardous for endovascular treatment. Inferior wall M1 aneurysms arise at the origin of the anterior temporal artery or the temporopolar artery and project toward the temporal lobe in an anterolateral projection.
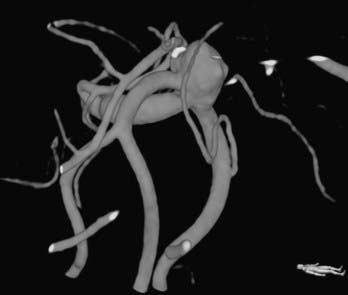
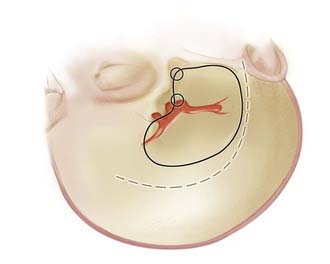
Bifurcation and trifurcation aneurysms represent up to 90% of all MCA aneurysms and are the lesions most uniformly referred for surgical consideration (Fig. 370-1). As noted in the previous brief discussion of MCA anatomy, the bifurcation (or trifurcation) can be highly variable, classically with one division supplying the frontal lobe and another supplying the temporal lobe. In a large Finnish series of MCA aneurysms, the direction of MCA bifurcation aneurysms varied considerably. Thirty-four percent were directed inferiorly in both the lateral and anteroposterior planes.6 With three-dimensional rotational digital subtraction angiography (DSA) and computed tomographic angiography (CTA), the precise orientation of the aneurysm relative to the vascular and cranial anatomy can be imaged preoperatively.
Classification by Etiology
Saccular
With regard to etiology, the precise pathogenesis of the common saccular (or berry) aneurysm is incompletely understood. These aneurysms classically form at sites of arterial curves or branching.21 Hemodynamic forces are likely to be an important contributing factor in the forced segmentation of the arterial elastic membrane, which may be an important factor in the aneurysm formation cascade.22
Fusiform Aneurysms
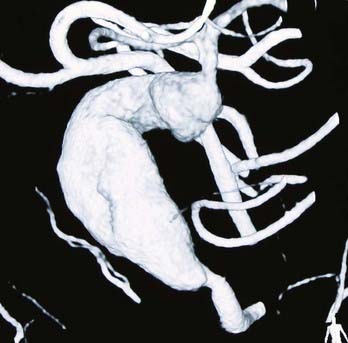
Fusiform lesions are most frequently seen in the posterior circulation and have far different management considerations than saccular aneurysms (Fig. 370-2). Although rare,6 there is no doubt that they represent a different disease process than saccular aneurysms, and in the MCA distribution, they can grow quite large, incorporating multiple branch vessels. MCA fusiform aneurysms can be found in all segments of the artery, although classically they are found at the bifurcation.
Infectious Aneurysms
Infectious or mycotic aneurysms are most commonly found along the distal branches of the cerebral arteries.23–25 They are typically secondary to infectious emboli, with subsequent aneurysm formation. Usually, bacterial endocarditis (65%) is implicated in infectious aneurysms, but other idiopathic bacterial or fungal sources have been implicated.23,26 Other embolic sources include neoplastic disease, such as choriocarcinoma and atrial myxoma.
Dissecting Aneurysms
Dissecting MCA aneurysms are rare and may be associated with infection, connective tissue diseases such as Marfan’s syndrome, cystic medial degeneration, and fibromuscular dypslasia.23,27–30 It is assumed in such scenarios that there exists a congenital weakness of the vessel wall with rupture of the elastic interna.30,31 The dissection is classically located between the internal elastic lamina and medial layers, which is different from dissecting aneurysms of the aorta or peripheral arteries.30 The intima is folded and displaced by the second lumen, causing the true lumen to be narrowed or completely blocked. Angiographically, if flow is present, there is an asymmetric narrowing32 or rippled appearance due to the separated or folded intima.30 The pathologic findings make the treatment of this rare disease process challenging. If infarction has been avoided and patency has been preserved, trapping with bypass or endovascular stenting, or both, may be considered. Although most present with ischemia, 43% of patients with an MCA aneurysmal dissection present with SAH, and 75% of these patients are male.33
Traumatic Aneurysms
Major cranial trauma is often associated with intracranial arterial dissection.25,34–36 Traumatic aneurysms are uncommon and are most often associated with the ACA and its branches because of its proximity to both the skull base and the falx. Traumatic MCA aneurysms are unusual, are most classically associated with a skull fracture, and have a high rupture rate.37 These lesions are most frequently distal on M3 and M4 segments and often present with delayed rupture (average, 4.7 days) after the inciting trauma.37 Classically, these are managed with surgical trapping and excision with or without bypass.
Classification by Size
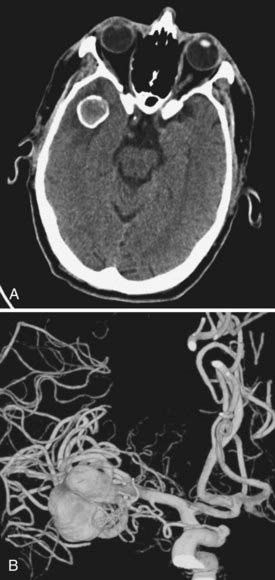
Cerebral aneurysms that reach large (>15 mm) or giant (>25 mm) size are more frequently seen in the middle cerebral artery distribution than in other arterial distributions and can represent up to 9% of MCA aneurysms.6 As with smaller aneurysms, they come in two types, saccular and fusiform, but because of their size, they represent significant treatment dilemmas (Fig. 370-3). Although often asymptomatic, they can also present with symptoms associated with mass effect or with transient ischemic attacks (or stroke) due to thrombus or flow reduction. About half have a neck that is amenable to clipping.2 Advanced surgical management may include clip reconstruction, bypass, wrapping, aneurysmorrhaphy, or combination techniques, including surgical and endovascular management. Some lesions are best managed by expectant management or regional referral, depending on surgeon and patient preferences.
Presentation and Evaluation of Middle Cerebral Artery Aneurysms
Subarachnoid or intracranial hemorrhage is the most common presentation of MCA aneurysms. Because of their propensity to become quite large before detection, they may occasionally become symptomatic without SAH. Giant aneurysms are reported to cause seizures more often than smaller ones, and this may be due to mass effect, ischemic changes, or repeated subclinical hemorrhages.38–41 The evaluation and treatment of patients with aneurysms varies depending on whether an aneurysm has ruptured or not. Nonruptured aneurysms are increasingly being discovered incidentally because of the frequency of use of screening studies (computed tomography [CT], CTA, magnetic resonance angiography [MRA]) in the evaluation of any number of unrelated symptoms. Occasionally, patients with a strong family history of aneurysms undergo screening as well. Unless treatment is not likely to be eventually recommended, we generally evaluate these patients with a catheter angiogram to fully assess the aneurysm, its morphology, and the remaining cerebral vasculature.
In symptomatic cases, CT usually reveals the presence of subarachnoid blood; however, a lack of blood necessitates a lumbar puncture (LP) if the history is suggestive of an aneurysm rupture.42 In patients with SAH on CT or LP, we typically perform a cerebral angiogram, with three-dimensional reconstruction of any pathology if possible.
Surgical Treatment of Middle Cerebral Artery Aneurysms
MCA aneurysms tend to be highly variable with regard to their ease or difficulty of surgical clipping. Variables include presence of SAH, size and configuration of the aneurysm, inclusion of branch vessels, and surgeon’s experience. Because of their frequently broad-based configuration, many MCA aneurysms are unsuitable for current endovascular options. In addition, the relatively small caliber of surrounding branches often precludes the use of stents, and their classic bifurcation location makes recurrence more likely. For all these reasons, and the fact that the lesions are usually readily accessible surgically, MCA aneurysms are preferentially clipped at most cerebrovascular centers.6,43,44
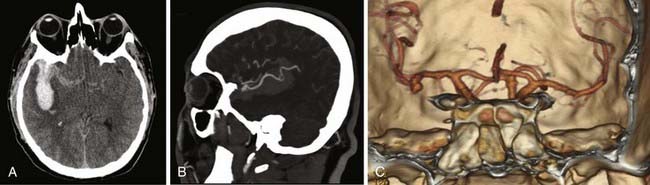
We attempt to treat ruptured MCA aneurysms at the first available opportunity.45,46 Treatment is generally scheduled for the next available operative day, ideally before postbleed day 3. Occasionally, patients are delayed in transfer and arrive with significant vasospasm as documented on DSA or CTA. In these situations, we have found it prudent to wait until after the vasospasm window, classically postbleed days 11 to 14, and manage these patients expectantly in the intensive care unit. Occasionally, but classically, MCA aneurysms can present with a large intracerebral hemorrhage that requires urgent or emergent clot evacuation and exploration and treatment of the aneurysm (Fig. 370-4). Unruptured MCA aneurysms that present incidentally are counseled regarding the various management options. Age of the patient, size and multiplicity of the aneurysm, family history, tobacco use, control of hypertension (if present), overall medical condition, and patient and family wishes are considered and discussed, using our knowledge of the natural history as a guide.47,48
Preparation
Cerebrospinal fluid drainage is often indicated in patients with acute hydrocephalus after aneurysmal SAH. Although there are anecdotal reports of rupture, several small studies have not confirmed an increased risk,49,50 and the relaxed brain makes surgery safer in ruptured aneurysm surgery. However, in patients with good-grade (Hunt-Hess grades I and II) disease, we often defer placement of a ventriculostomy, or may consider placement of a lumbar drain. Patients with unruptured aneurysms generally do not have preoperative placement of a lumbar drain. Most patients receive a bolus of mannitol at the beginning of the operation to assist in brain relaxation.
As with all cranial surgery, the positioning of the incision and head are of paramount importance and cannot be overlooked. A radiolucent cranial fixation device is used in all cases to assist with intraoperative angiography. For MCA aneurysms, the head is turned about 30 degrees to the opposite side with the vertex slightly extended (Fig. 370-5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree