Chapter 34 Surgical Management of Midline Anterior Skull Base Meningiomas
Surgical Anatomy
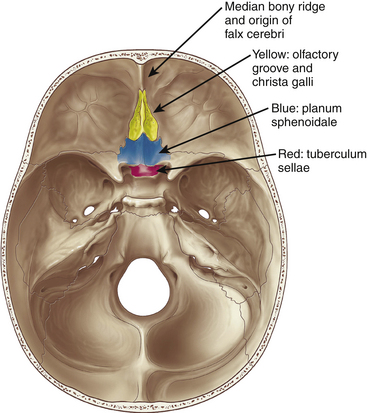
Meningiomas arising in the midline of the anterior fossa are generally separated in the more ventral olfactory groove meningiomas and the more dorsal planum sphenoidale and tuberculum sellae meningiomas. Olfactory groove meningiomas arise over the cribriform plate of the ethmoid bone and the area of the frontosphenoid suture. Those tumors may grow symmetrically around the crista galli and thus may involve any part of the planum of the sphenoid bone or extend predominantly to one side. They occurred with a frequency of less than 6% in our series of 1200 meningiomas. Of all anterior skull base meningiomas, 22% were pure olfactory groove meningiomas. Of these, 7% had at least one additional meningioma at a different location. Planum sphenoidale/tuberculum sellae meningiomas arise from the roof of the sphenoid sinus and the tuberculum sellae, which is an area between the optic nerves and the anterior clinoid processes belonging to the frontal part of the middle cranial fossa. The tuberculum sellae is located between the chiasmatic grooves and on either side at the optic foramen, which transmits the optic nerve and ophthalmic artery to the orbit. Behind the optic foramen, the anterior clinoid process is directed posteriorly and medially and attaches to the tentorium cerebelli. These structures are frequently overgrown by these types of meningiomas, as are the posteriorly located dural folds of the sella turcica and the lateral adjacent cavernous sinus (Fig. 34-1). Planum sphenoidale/tuberculum sellae meningiomas occurred at rates similar to those of olfactory groove meningiomas in our series: less than 6% of all intracranial meningiomas but 21% of anterior skull base meningiomas.
Clinical Presentation
Olfactory groove meningiomas are on average larger than meningiomas at different locations in our series. This is most likely due to the relative lack of focal symptoms at the frontal base with smaller meningiomas. For large tumors, the slow growth rate allows surrounding tissues to adapt. Many symptoms are difficult to localize neurotopically, and the initial consultations of family and physicians often tend toward interpretation of these as functional personality changes rather than focal cerebral symptoms. Personality changes, such as apathy and akinesia, can be common when the tumors grow to larger size; in our series, this was found in up to 13% of patients. Onset of these symptoms is gradual, and they may not be observed early in their course. Other common symptoms include headache and visual deficits, both of which were more frequent in frontal meningiomas than in any other type of meningioma in our series. Because the optic nerves and chiasm are compressed superiorly by the tumor, an inferior visual defect was most common in up to one third of our patients. The Foster-Kennedy syndrome of unilateral optic atrophy and contralateral papilledema, although originally described in olfactory groove meningiomas,1 occurred in only a small number of patients.
Double vision is a rare symptom, occurring in less than 6% of patients. In our series, smelling disorders up to anosmia were apparent in 64.5% of the patients that were diagnosed based purely on the routine preoperative workup. Only 7.1% were completely anosmic preoperatively. Interestingly, anosmia is not an important symptom for most patients, most likely because it develops slowly. In Cushing and Eisenhardt’s series, the sense of smell was the primary symptom in only 3 of the 29 patients.2 Bakay reported that even if anosmia was apparent, it was not the leading symptom.3
Evaluation of Radiologic Studies in Planning the Operation
In recent years, angiography generally has not been indicated unless embolization is planned. The classic angiographic appearance of a meningioma is that of increasing hypervascular tumor blush throughout the arterial phase, persisting well into the late venous phase with slow washout. Hypervascularity may complicate and lengthen the operation. Therefore, embolization may be considered, which involves the devascularization of the tumor’s blood supply through the placement of an embolic agent via a microcatheter into the feeding arteries. However, the surgeon should be aware of a considerable rate of hemorrhagic and ischemic complications when using small particles for embolization.4–7
General Aspects of Surgical Management
As the majority of meningiomas are benign, well-circumscribed extra-axial tumors, complete surgical removal should be the primary goal in most instances. The neurologic integrity has to be preserved as it would be in any other neurosurgical procedure. Due to the relation of anterior midline meningiomas to adjacent neurologic eloquent areas, complete tumor removal even with resection of infiltrated dura or removal of infiltrated bone might be achieved with low morbidity in most cases. However, when tumors are firmly attached to the anterior vessels or the optic chiasm, complete removal might constitute a high risk for damage of those structures. In these cases a small piece of adherent capsule might remain and is controlled by periodic MRI scans. Upon recurrence, reoperation and adjuvant radiation therapy may be considered.
Bifrontal Approach
General Considerations
The bifrontal approach was first described by Horsley8 and Cushing9 and was later proposed by Tönnes,10 who preserved the frontal brain tissue by a subfrontal approach.11 Many others have used the bifrontal approach for large tumors of the frontal base, such as Al-Mefty,12 Nakamura et al.,13 and Ransohoff and Nockels.14 A bifrontal craniotomy might be considered for patients with large tumors because this approach gives direct access to all sides of the tumor. Due to the wide exposure, retraction on the frontal lobes is minimal. It simultaneously allows interruption of the blood supply, preparation of the frontobasal matrix of the tumor, and concomitant decompression. There is usually no problem from the ligation of the anterior sagittal sinus. However, venous drainage should be evaluated by preoperative imaging to avoid venous congestion, and coagulation of draining veins from the anterior frontal lobe should be avoided of possible. A navigation system might be used to avoid opening the frontal sinus; however, if the frontal sinus is entered, meticulous closure of the defect should prevent any complications.
Operative Technique
The patient is placed in the supine position with the knees slightly flexed and the head slightly elevated and extended. A three-point skeletal-fixation headrest system is used. Usually, only a small area of hair needs to be shaved to prepare a coronal incision through the skin while preserving the pericranial tissue. The skin of the posterior aspect of the incision is elevated, and the pericranial tissue is incised below the skin. This step gives extra pericranial tissue, which might be used later to cover the floor of the anterior fossa and to patch the convex dura as needed. The skin flap and underlying tissue, including the pericranial tissue, are then turned down together using fishhooks. According to the size and extension of the tumor, bur holes are placed just below the end of the anterior temporal line or keyholes are placed above the pterion and on each side of the sagittal sinus anterior to the skin incision (Figs. 34-2 and 34-3).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree