Chapter 32 Surgical Management of Parasagittal and Convexity Meningiomas
Epidemiology and Significance
Meningiomas are the most common primary brain and central nervous system tumor (incidence rate, 6.29 per 100,000 persons). Data from the 2004–2006 Central Brain Tumor Registry reveal that these tumors account for approximately 34% of all primary brain tumors. Meningiomas occur in females 2.25 times more frequently than males (incidence: 8.44 per 100,000 females vs. 3.76 per 100,000 males).2 An increased incidence has been reported among Africans and black Americans.3,4 The incidence rate increases with older age and peaks in persons over 85 years of age (incidence over age of 85 years of age: 36.9 cases per 100,000 persons).2 Meningiomas occur most frequently on the convexity (19% to 34%) and parasagittal locations (18% to 25%), followed by the sphenoid wing and middle cranial fossa (17% to 25%), anterior skull base (10%), posterior fossa (9% to 15%), cerebellar convexity (5%) and clivus (<1%).5–7 To optimize patient management and surgical outcome, understanding appropriate surgical objectives and technique based on the location and biology of these neoplasms is critical.
Etiology
Sporadic
The etiology of the majority of meningiomas is unknown. Although head injury, viral infection, and cell phone use have been implicated in the development of intracranial meningiomas,8,9 the data are inconclusive and conflicting for each of these potential etiologies. The strongest support for an etiologic role in the development and progression of sporadic meningiomas is hormonal. Specifically, studies have shown a potential progesterone influence in the development of meningiomas based on the propensity of these tumors to occur in females and the presence of progesterone receptors in the majority of meningiomas.10–13 While few definitive etiologic correlations have been made for meningiomas, there are certain iatrogenic, environmental exposure and genetic causes that have been linked to their development. These tumorigenic etiologies have significant implications for the management of a meningioma patient who presents for treatment.
Radiation Induced
Meningioma development is associated with irradiation exposure. This association was established by two large cohorts of individuals exposed to radiation that subsequently developed tumors, including meningiomas. Studies published on a group of nearly 11,000 Israeli children treated with low-dose (approximately 1.5 Gy) radiation for tinea capitis revealed a 9.5 times risk for development of meningioma compared to matched controls and untreated siblings.14,15 Similarly, the incidence of meningioma formation among 68 survivors of the Hiroshima atomic bomb within a 2-km radius of the hypocenter identified a 2.9 times risk for the development of meningioma with higher risk for patients within a 1-km radius (6.7 times risk).16 Other iatrogenic forms of ionizing radiation exposure have also been linked to the subsequent development of intracranial meningiomas, including higher-dose, full-mouth dental radiographs and radiotherapy provided for treatment of other cancers.17–20 Generally, radiation-induced meningiomas have a dose dependent latency to development of 10 to over 30 years and have a higher propensity to recur after resection.21
Radiation-induced meningiomas tend to be a higher pathologic grade when compared to their sporadic counterparts, but even WHO grade I radiation–induced meningiomas exhibit more aggressive clinical behavior and have an increased proclivity to recur when compared to their sporadic counterparts.19,22,23 Patients with radiation-induced meningiomas also have an increased incidence of multiple tumors, which must be accounted for when planning placement of the incison.17,24,25 Further, atrophic changes in the scalp that commonly accompany cranial irradiation can require modification of standard multilayer scalp closure. Because radiation exposure can result in other delayed side effects, patients with radiation-induced meningiomas require close medical follow-up for other sequelae, including pituitary dysfunction, visual disturbances (optic atrophy), radiation necrosis, and development of other neoplasms (sarcoma, glioma).26
Neurofibromatosis Type 2 (NF2)
NF2 is an autosomal dominant heritable tumor predisposition syndrome that leads to the development of central and peripheral nervous system tumors (meningiomas, schwannomas, ependymomas), ophthalmologic findings (cataracts, epiretinal membranes, retinal hamartomas) and cutaneous findings (skin plaques, subcutaneous tumors).27 Intracranial meningiomas are identified in approximately half of NF2 patients and are a significant source of morbidity and mortality. NF2-associated intracranial meningiomas are frequently multiple and develop at a younger age than compared to sporadic cases of meningiomas.28–31 Up to 20% of children presenting with a meningioma will have NF2, necessitating full clinical screening and longitudinal follow-up.28,32 The presence of intracranial meningiomas is associated with a 2.5-fold rise in relative risk of mortality in patients with NF2.33 Meningiomas associated with NF2 frequently have increased proliferative activity and a greater rate of atypical and anaplastic grades than do sporadic meningiomas.34,35 Because of the frequent multiplicity of lesions, we perform resection of tumors based on the development of symptoms rather than radiographic tumor growth.
Multiple Meningiomas
Examination of a large series reporting on the presence of multiple meningiomas found that 1% to 10.5% of patients may present with multiple tumors.34 Identification of patients with multiple meningiomas has increased significantly with the advent of improved imaging techniques. Both sporadic and familial forms of multiple meningiomas have been described in the literature, independent of NF2 or history of radiation exposure.37–40 Familial forms of the disease follow an autosomal dominant inheritance pattern and are caused by a mutation independent of the NF2 gene.41,42 Tumors in patients with multiple sporadic meningiomas appear to originate from a single clone (suggesting metastatic spread), whereas others may develop these tumors independently, based on cytogenetic differences among tumors.43–45 Patients with sporadic or familial forms of multiple meningiomas present dilemmas in management similar to patients with NF2, resection is often reserved until the development of symptoms rather radiographic tumor growth.
Classification
Meningiomas originate from arachnoid cap cells, which are distributed along the entire neuroaxis and reflect the wide spectrum of tumor localization. The preoperative classification of meningiomas is based on location of the tumor, primary dural attachment and relationship to neurovascular structures. In the Cavendish Lecture presented in 1922, Harvey Cushing coined the term “meningioma” and classified these tumors based on their site of origin.1 Later, in 1938, Cushing and Eisenhardt used preoperative anatomic classification of meningiomas as a method to correlate clinical findings, help determine surgical approach and aide in developing a prognosis.46 To this day, preoperative anatomic localization remains of critical importance, because it determines surgical positioning, placement of incision and risks involved with resection of the tumor.
Convexity Meningiomas
Convexity meningiomas refer to tumors of the supratentorial space that have their sole attachment to the dura covering the convexity of the cerebral hemispheres. The original classification of convexity meningiomas by Cushing included temporal, frontal, paracentral, parietal and occipital locations.1 Several studies examining the distribution of convexity tumors reveal that the frontal region is the most common site of origin (over 50%), with the posterior locations least frequent (7% to 11%), and the remainder equally distributed between the temporal and paracentral locations.47,48 Because of improvements in the ability to localize tumors and their relationship to functional cortices based on anatomic and functional imaging studies, it is possible to preoperatively classify convexity tumors, anticipate clinical symptoms and the specific risks associated with their management.
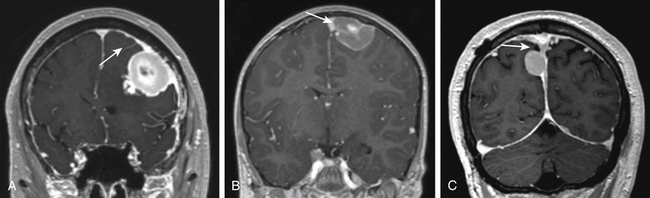
Parasagittal Meningiomas
Parasagittal meningiomas have an attachment to the dura forming the outer layer of the SSS and occupy the parasagittal angle displacing brain from this location (Fig. 32-1). Olivecrona first classified parasagittal meningiomas based on their anatomic location along the superior sagittal sinus (SSS). He divided the SSS into thirds anatomically (anterior, middle, posterior) because of the potential consequences of sinus occlusion during complete removal of meningiomas in each area.49 The most common location for parasagittal meningiomas to arise is along the middle third of the SSS (from coronal suture to bregma). Tumors in this location account for 37% to 70% of parasagittal tumors. Fifteen to 42% of parasagittal meningiomas are located along the anterior third of the SSS (from the glabella to the coronal suture) and 9% to 16% of parasagittal tumors are located along the posterior third of the SSS (between the bregma and torcula).50,51
Differentiating parasagittal meningiomas from convexity and falcine meningiomas has significant implications for preoperative evaluation and surgical planning. In the instance where tumors of the convexity approach the SSS, they are distinguished from parasagittal tumors by the presence of brain tissue at the parasagittal angle and lack of attachment to the meninges that create the outer wall of the superior sagittal sinus itself. A similar distinction is made of falcine meningiomas, which are delineated from parasagittal tumors because their sole dural attachment is the falx cerebri. Parasagittal meningiomas vary in the amount of attachment to convexity dura and the falx cerebri, but they all have an attachment to the dura forming the outer layer of the SSS and occupy the parasagittal angle displacing brain tissue from this location (Fig. 32-1).
Clinical Presentation
Meningiomas are discovered incidentally or as a result of the development of related signs and/or symptoms. The increasing use of computed tomography (CT) and magnetic resonance (MR)-imaging has led to an increase in the number of incidentally discovered meningiomas over the last two decades. Currently, incidentally discovered meningiomas represent 10% to 20% of all meningiomas that are brought to clinical attention. Overall, clinical symptoms from meningiomas develop as a result of raised intracranial pressure, disruption of cortical electrophysiology or direct mass effect on adjacent neural structures.
Convexity Meningiomas
Similar to other mass-occupying lesion in the central nervous system, convexity meningiomas can present with a variety of signs and symptoms based on their anatomic location. Symptomatic convexity meningiomas most commonly are associated with headache (39% to 48%), seizures (20% to 34%), and/or hemiparesis (10% to 21%).47,48,52 Other specific symptomatology, such as dysphasia, sensory changes, and visual disturbance occur less frequently but develop when tumors are located specifically over the respective eloquent cortices.
Parasagittal Meningiomas
Signs and symptoms associated with parasagittal meningiomas depend on their location along the SSS. Tumors of the anterior third of the SSS often cause headache and personality changes. Tumors of the middle third of the SSS often are associated with Jacksonian seizures, headache and progressive hemiparesis. Tumors of the posterior third of the SSS often cause headache, seizures and gradual hemianopsia.50,51 Like convexity tumors, the most common symptoms associated with parasagittal meningiomas at the time of presentation are seizures (46% to 51%), headache (42% to 54%), and/or motor weakness (39% to 49%).
Indications for Surgery
Incidental Meningiomas
Because they exhibit no growth or slow linear growth, the majority of asymptomatic, incidental meningiomas may be observed without surgical intervention.53,54 Several studies reporting on the long-term natural history of these tumors also reveal that a smaller subset of tumors may demonstrate exponential growth or sigmoidal growth.55,56 Variability in growth rates and patterns necessitate close surveillance until these properties can be established.57 We perform follow-up MR-imaging 3 months after initial diagnosis for incidentally discovered meningiomas. If the tumor demonstrates no growth or slow linear growth, we will monitor the tumor at 6 to 12 month intervals. Alternatively, we monitor tumors exhibiting exponential growth at 3 to 6 month intervals. Specific imaging and patient characteristics associated with more rapid meningioma growth rates have been identified. The likelihood of tumor growth is higher in young patients and tumors larger than 3 cm in size.53,58,59 Tumors that lack calcification and are hyperintense on T2-weighted MR-imaging are also more likely to display an aggressive growth pattern.55,57,60 For each clinical situation, the decision to recommend surgery should be evaluated on an individual basis, incorporating patient comorbidities, age, observed growth rate, and image-based predictive factors for growth.
Parasagittal Meningiomas
In addition to the indications presented for convexity tumors, timing of parasagittal meningioma resection is influenced by the extent of SSS involvement. Because acute surgical obstruction of the patent SSS can cause significant cerebral edema and venous infarction, a tumor causing partial obstruction may be followed closely to observe for the development of complete occlusion, allowing that the intracranial component of the tumor does not greatly increase in size, cause symptoms, or encroach on large adjacent superficial anastomosing veins. Alternatively, an asymptomatic meningioma without evidence of invasion into the SSS may require intervention at the earliest sign of growth so that a complete resection can be performed without entering the SSS.
Preoperative Evaluation
Superior Sagittal Sinus Involvement
With advancements in imaging and microsurgical techniques, reconstruction and bypass of the SSS have been used in an effort to enhance resection parasagittal meningiomas. Several large series have been published on the complete removal of tumor with reconstruction or bypass of the SSS.61 Despite performing more extensive resections (Simpson grade I, Table 32-1) with sinus reconstruction or bypass, which increased perioperative morbidity and mortality, the authors found that local recurrence remains a significant problem.62 Subsequently, based on the potential morbidity with these cases, better understanding of the natural history of meningiomas and efficacy of adjuvant radiosurgery63–65 in the management of residual and/or recurrent disease, many surgeons have adopted a more conservative approach to management of SSS invasion by meningioma.62,63,66
Table 32-1 Simpson Classification of Extent of Resection for Intracranial Meningiomas and Recurrence Rate
| Grade | Extent of Resection | Recurrence Rate |
|---|---|---|
| I | Gross total resection of tumor, dural attachments and abnormal bone | 9% |
| II | Gross total resection of tumor, coagulation of dural attachments | 19% |
| III | Gross total resection of tumor without resection or coagulation of dural attachment or its extradural extensions | 29% |
| IV | Partial resection of tumor | 44% |
| V | Simple decompression |
Derived from a surgical approach that does not include resection and repair/reconstruction of a patent or partially occluded SSS, a simple classification system can be used that permits stratification of parasagittal meningiomas based on preoperative imaging (Fig. 32-2). This classification scheme divides parasagittal tumors into three types that have direct surgical implications. Type I tumors are defined as tumors that attach to the external layer of dura forming the SSS. Type II tumors are identified as tumors that visibly invade the SSS and narrow its lumen, but do not cause complete obstruction. Grade III tumors are defined as tumors that invade the SSS and cause its complete obstruction. Each of the three types has surgical implications as defined below.
The evaluation of SSS patency, invasion and the development of collateral venous pathways are performed with contrast-enhanced MR-venography (CE-MRV). The sensitivity and specificity for these findings on CE-MRV is similar to that of digital subtraction angiography (DSA), with a reduction in risk and patient discomfort.67,68 DSA is still performed if tumor embolization is considered or if arterial supply to the tumor needs to be better defined. In the setting of SSS obstruction, CE-MRV, and DSA may reveal significantly dilated scalp veins and diploic veins in addition to engorged cortical venous collaterals. These supplementary collateral venous pathways play a critical role in venous drainage, especially in the setting of parasagittal meningiomas causing occlusion of the middle or posterior third of the superior sagittal sinus. When possible, surgical approaches should avoid transgression of these structures with tailored scalp incisions and bone flaps.62,69 Although convexity and parasagittal meningiomas typically receive their primary blood supply from dilated meningeal arteries, the surgeon should be aware that some tumors may develop additional blood supply through the internal carotid artery (pial parasitization) or dilated scalp arteries (Fig. 32-3).
Bony Changes
Preoperative evaluation of bone involvement is useful in assessing the need for bone removal and cranioplasty. Because MR imaging alone significantly underestimates bony changes associated with meningiomas, we routinely perform a cranial CT for evaluation of the extent of bony involvement of all parasagittal and convexity meningiomas.70 Bony changes associated with meningiomas can be caused by hyperostotic changes or direct bone invasion by tumor. Hyperostotic changes are generally considered a benign form of inductive ossification caused by tumoral increases in alkaline phosphatase production.71 In managing hyperostotic changes without bony invasion by tumor, we use the high-speed drill to remove the bulk of hyperostotic bone, at times just leaving the outer cortical table of bone intact. For tumors that present with significant bone destruction and replacement by tumor, removal of the invaded bone is necessary for complete tumor resection.
Tumor and Brain Characteristics
The “gold standard” for evaluation of meningioma size, location, and impact on adjacent brain structures is MR-imaging performed with and without contrast. Meningiomas typically appear as isointense masses on pre-contrast T1-weighted imaging and enhance homogenously after the administration of contrast. Postcontrast MR imaging often reveals a trailing linear enhancing structure along its dural attachment referred to as the “dural tail.”72 The dural tail is not specific to the diagnosis of meningioma, but its management continues to be a point of controversy.72,73 Although evidence suggests that most of the dural tail is an imaging correlate of dilated meningeal vessels and dural congestion,74 many still advocate extensive resection of the dural tail believing that it can contain tumor. Generally, we resect at least a 1-cm margin of dura from its interface with the tumor.
T2-weighted MR-image sequences typically show more variability in tumoral intensity (50% isointense, 40% hyperintense, and 10% hypointense),75 but may help predict clinical behavior of meningiomas. Identification of a T2-weighted hyperintense arachnoid cleft between tumor and brain is often indicative of a distinct anatomic plane (Fig. 32-4). Absence of an arachnoid cleft in combination with significant peritumoral edema may be indicative of pial vessel parasitization and/or brain invasion. Addressing brain invasion is a key aspect in the operative management of parasagittal and convexity meningiomas. Management balances preoperative expectations, age of the patient, and eloquence of the involved cortices. If extrapial resection is not possible in an area of eloquence, a small amount of tumor may be left attached to the cortical surface and observed postoperatively.76 For tumors near eloquent areas with variable representation, such as Broca’s and Wernicke’s area, fMR imaging or awake surgery with mapping is valuable in elucidating their anatomic relationship and determining risks of resection.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree