25
Surgical Management of Pediatric Posterior Fossa Tumors
Following accidents, the leading cause of death in children is cancer. The most common solid cancer in childhood is medulloblastoma. That being said, the incidence of brain tumors in children is 2.6 to 4 per 100,000.1 It is thus no wonder that the average pediatrician, seeing 40 to 60 patients per day in his or her office, may overlook the possibility of a brain tumor in the child who presents with vomiting or dizziness early in the clinical course. In fact, the average child diagnosed with a brain tumor has been to three physicians and has been symptomatic for 6 months by the time the diagnosis is made. In an unpublished review of our earlier series, the author found that 24% of children diagnosed with a posterior fossa tumor had had an upper gastrointestinal (GI) series as part of their workup for vomiting prior to the diagnosis of a brain tumor.
Half of the brain tumors found in childhood occur in the posterior fossa, with medulloblastoma, juvenile pilocytic astrocytoma (JPA), and ependymoma being the big three.2 Multiple reviews of outcomes of children with posterior fossa tumors have demonstrated that, regardless of histology, the extent of resection is the most important predictor of outcome. In trials of ependymoma and medulloblastoma, a gross total resection or near-total resection doubles the 5-year survival of the child compared with a lesser resection.3–5 Thus, the work we do as neurosurgeons is more important to the survival and the quality of the survival of the child with a posterior fossa tumor than anything the radiation oncologist or the medical oncologist can add to the care. There are also studies showing that a fellowship-trained pediatric neurosurgeon is more likely to obtain a gross total resection of a child’s posterior fossa tumor than a generalist. It is paramount that the neurosurgeon who decides to operate on a child’s posterior fossa tumor be prepared to completely excise the tumor if feasible. To do so safely requires not only a skilled neurosurgeon but also a skilled pediatric anesthesiologist and skilled pediatric intensivists to assist with postoperative care. If this team is not available, it is better to refer the child to a specialty center. Fifty percent of the children referred to us with posterior fossa tumors have undergone inadequate surgery and have to undergo a second posterior fossa surgery to optimize their outcome.
Whereas in years past there were few textbooks to assist the neurosurgeon in preparing for surgery in the infant or child, there are now several good reference texts that can be referred to for specific tumors and their management.6–9
♦ Clinical Presentation
The mean age of presentation of children with medulloblastoma or ependymoma is 5 years or less, whereas the mean age of presentation of the child with a juvenile pilocytic astrocytoma is 9 years. Furthermore, children with malignant brain tumors are more likely to present with weight loss and constitutional symptoms as compared with children with benign tumors. Remember that a 2- to 4-pound weight loss in a 5-year-old may represent 10 to 15% of the body mass and is significant. Finally, children with malignant tumors are generally symptomatic for a shorter period of time than are children with benign tumors. The exception to this is the child with a large cystic component to a pilocytic tumor or a hemangioblastoma, as these cysts can sometimes enlarge at a rapid rate.
Infants with posterior fossa tumors present with a tense fontanelle and progressive head growth. It is surprising how often a head crossing percentiles on a growth curve is the only clinical clue of an underlying posterior fossa tumor. As the tumors progress, the infants become irritable, have associated vomiting, and may have altered sensorium. They may at times present in extremis, with decerebrate posturing or coma.
Once children reach an age where they can communicate and ambulate, they typically present with morning headaches and vomiting and gait ataxia. They are commonly referred to the ear, nose, and throat (ENT) department for evaluation of vertigo or labyrinthitis. Papilledema and the inability to walk tandem are common clinical signs. If the vermian tumor is eccentric, they may have dysdiadochokinesia as well. Our practice is to image the entire spine preoperatively in children with posterior fossa tumors, as postoperative imaging of the spine may be artifactual for weeks thereafter. In the child who has back pain, tenderness, or hyperreflexia, consideration of drop metastases from a malignant tumor should be entertained. Similarly, if the child with a posterior fossa tumor presents with seizures, cortical metastases or leptomeningeal disease should be considered.10
♦ Imaging
At the present time, there is no imaging modality that can predict the histology of a posterior fossa tumor with certainty. We have seen densely enhancing nodules associated with large cysts that were presumed to be pilocytic nodules and yet at surgery turned out to be hemangioblastomas (Fig. 25.1). We have seen multicystic tumors that we thought were JPAs but proved to be medulloblastomas. We have seen tumors with calcifications that we thought would be ependymomas turn out to be pilocytic astrocytomas. Having said this, there are some clues that can help predict what the tumors will most likely turn out to be. Our experienced neuroradiologists are able to predict tumor histology in nine out of 10 cases. Tumors that appear to be hyperdense on noncontrasted computed tomography (CT) are most likely to be primitive neuroectodermal tumors (Fig. 25.2), whereas the ependymomas and astrocytomas are hypodense on noncontrasted CT. A large vermian tumor that extends out of the foramen of Magendie or extends into the cervical spinal canal is more likely be an ependymoma (Fig. 25.3). Likewise, a tumor of the fourth ventricle that grows out the foramen of Luschka and fills the cerebellopontine angle is likely to prove to be an ependymoma (Fig. 25.4).11
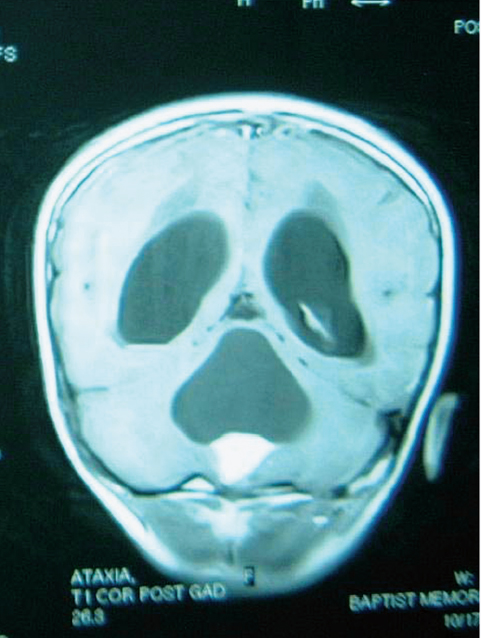
Fig. 25.1 Coronal T1-weighted magnetic resonance imaging (MRI) scan with gadolinium enhancement in a 6-year-old with a cystic pilocytic astrocytoma. At times, hemangioblastomas can give a similar imaging appearance of a densely enhancing nodule and nonenhancing cyst wall.
♦ Preoperative Management
Although these children present with ventricular obstruction and symptoms of raised intracranial pressure, the vast majority can be observed in an intensive care setting and do not require placement of an emergent shunt, third ventriculostomy, or external ventricular drain. Within 12 hours of initiating intravenous steroids, most children defervesce, and surgery can be performed on an elective basis. There are cases in which a child declines neurologically and has to be operated on emergently, but such cases are uncommon. Most children with posterior fossa tumors do not require permanent shunts. In our experience, only a fourth of the children with posterior fossa tumors ultimately require ventriculo-peritoneal shunts. The use of external ventricular drainage is determined at the time of surgery based on the turgor of the dura after the craniectomy. Again, the majority of children do not require external ventricular drainage even though the ventricles may appear quite enlarged on preoperative imaging.
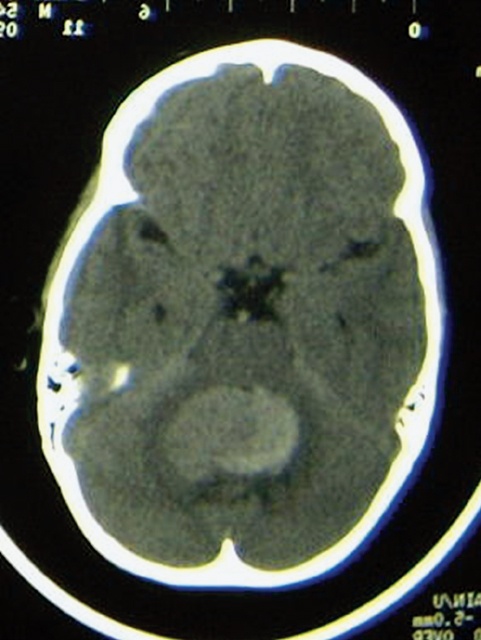
Fig. 25.2 Axial noncontrasted computed tomography (CT) scan of the posterior fossa in an infant with a medulloblastoma. Note that despite the lack of contrast, the tumor appears to be hyperdense. This is typical of highly cellular tumors such as permeative neuroectodermal tumors.
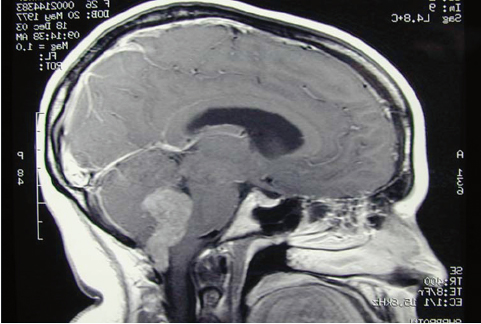
Fig. 25.3 T1-weighted sagittal MRI scan with gadolinium enhancement through the posterior fossa. The imaging appearance of this midline tumor extending out of the foramen of Magendie and into the cervical canal is typical of ependymoma; however, at the time of surgery this tumor proved to be a choroid plexus papilloma
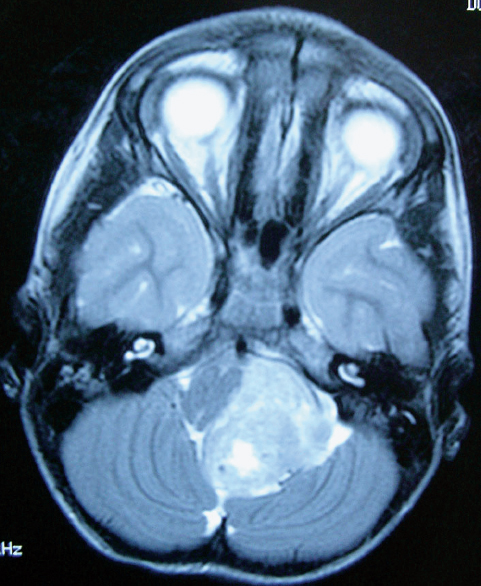
Fig. 25.4 Fluid-attenuated inversion recovery (FLAIR) axial MRI scan of the posterior fossa in a child with a cerebellopontine angle ependymoma. Note how the tumor displaces and deforms the brainstem and encases the vertebrobasilar junction. In such cases, a gross total resection is challenging but can be accomplished in 90% of cases.
♦ Operative Management
Several age-dependent factors enter into the decision making of positioning, anesthesia, and postoperative care. They are discussed in the following subsections.
Anesthesia
The greatest challenge of tumor surgery in children younger than 2 years of age is that of blood loss. The circulating blood volume of a young child is estimated at 70 cc per kilogram body weight. Loss of more than 1.5 blood volumes runs the risk of a coagulopathy. The anesthesiologist and the surgeon must both pay close attention to blood loss, particularly that hidden under the drapes or in drainage bags. The anesthesiologist should begin replacement early when it becomes apparent that a transfusion will be necessary. Washed red blood cells are less likely to cause intraoperative problems with hyperkalemia in the child requiring large volumes of blood. In children with malignant tumors who have been receiving chemotherapy, irradiated red blood cells may be given to reduce the likelihood of viral transmission to a compromised host.
For tumors of the cerebellar vermis, the surgeon should, just after opening the dura, dissect out the cerebellar tonsils, identify each posterior inferior cerebellar artery (PICA) as it loops below the tonsils, and dissect them rostrally until the vermian branches of the PICA are identified. These branches give the main blood supply to vermian tumors and can be ligated early in the procedure as they enter the tumor, thus dramatically reducing the vascularity of the tumor.
Positioning and Fixation
Most pediatric neurosurgeons have stopped placing children in the sitting position, as the Concorde (prone, neck flexed) position offers just as good an exposure with greater surgeon comfort and little risk of air embolism (Fig. 25.5). That being said, posterior fossa surgery in children younger than 2 is usually done with the patient face down on a horseshoe headrest, as the risk of pin fixation in the young skull is obviated. Because of this, the risk of pressure sores on the malar imminence or forehead requires meticulous attention. The authors place Rest-on Foam (3M Corporation, St. Paul, MN) over the face, with the adhesive side to the skin. This helps pad the face and also serves to anchor the endotracheal tube into place. For children over 3 years of age, the pediatric Mayfield pins are utilized but tightened to only 40 pounds of pressure until the pins grab the outer table of the skull.
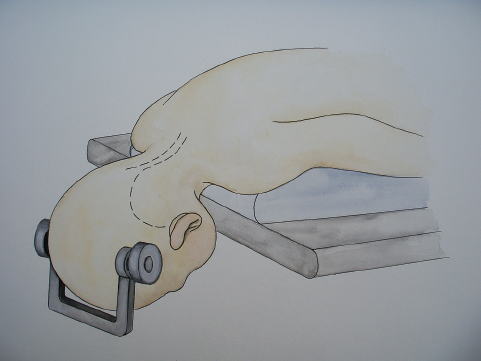
Fig. 25.5 Artist’s rendering of an infant in the Concorde position. We usually flex the patient’s neck to within a fingerbreadth of the chest. Care must be taken that the patient’s chin and face are well away from the table or any compressive objects to avoid pressure sores acquired during surgery. For children with cerebellopontine angle tumors, the neck is similarly flexed, but the chin is brought to the shoulder, turning the involved retromastoid region toward the surgeon. This approach facilitates exposure of the fourth ventricle as well as the lateral aspect of the brainstem.
Approaches
Although medulloblastomas can be found within the cerebellar hemisphere, most arise from the roof of the fourth ventricle, pushing downward into the ventricle; 35% invade the brainstem, often at the obex or the floor of the fourth ventricle. This can often be identified preoperatively by close inspection of the magnetic resonance imaging (MRI). Alternatively, upon exposing the cerebellum, the microscope is brought into the field and the cerebellar tonsils separated as the vermian branches of the PICA are dissected out. This approach facilitates concomitant inspection of the floor of the fourth ventricle. If it is invaded by tumor, caution must be taken in manipulation of the tumor as it is debulked, as this could lead to a “floor of the fourth syndrome” which includes an ipsilateral palsy of cranial nerves VI and VII and a contralateral hemiparesis.
Juvenile pilocytic astrocytomas arise from within the cerebellar hemispheres and are usually separated from the ventricle by the ependyma. On some occasions, however, they also invade the floor of the fourth ventricle. A variant of the cerebellar astrocytoma actually arises from the brainstem and is dorsally exophytic into the ventricle or out laterally into the cerebellopontine angle. Such tumors exit the brainstem, pulling functional tissue up with them much like the sides of a volcano. The inexperienced surgeon may be inclined to shave these tumors off flush with the floor of the ventricle or with the side of the stem, inadvertently injuring the brainstem in the process.
Ependymomas, by definition, take origin from the walls of the ventricle. They must carefully be debulked as the capsule is dissected away from neural tissue. Those arising from the floor of the fourth ventricle derive blood supply from multiple small perforating vessels arising from the brainstem. These vessels must be meticulously coagulated and cut, as avulsing them may cause them to retract and bleed into the brainstem. If they do, they should not be pursued. Gentle suction with a regulated suction, gentle irrigation, and time will allow them to stop oozing without injuring the brainstem.
A rare variant of the ependymoma arises from ependymal rests at the outer margin of the foramen of Luschka and grows out the foramen into the cerebellopontine angle as well as into the fourth ventricle. These tumors often grow to be quite large before obstructing the ventricle and causing hydrocephalus, their most common presenting symptom. By this time, these tumors often encase the lower cranial nerves as well as the vertebrobasilar complex, and may have invaded the side of the pons. This tumor is one of the most formidable posterior fossa tumors for the surgeon. Ependymomas have a predilection to grow out the porus acusticus or jugular foramen. To approach these tumors requires a modified Concorde position (Fig. 25.5) with the infant’s chin tucked and turned over to the shoulder as the infant is flexed. The skin incision begins midline but curves up behind the ear on the involved side. This allows bony removal across the midline, up to the torcular Herophili and around to the sigmoid sinus of the involved side. By gently elevating the involved cerebellar hemisphere and opening the telovelar space (cerebellomedullary fissure), one can dissect out the entire tumor and accomplish a gross total resection. Over half of these children require temporary tracheostomies and gastrostomies, but our experience has been that most of them can be decannulated by 6 months postoperatively.
In caring for children with phakomatoses, such as von Hippel-Lindau disease, one must recognize that the child will likely have recurrent disease elsewhere in the posterior fossa over time. As such, the surgeon performing the skin incision and approach to a single tumor should keep in mind the need for other approaches over time, as new tumors arise. One of three entries into the posterior fossa will, over time, allow access to the entire posterior fossa (Fig. 25.6).
Intraoperative Monitoring
Space limitations preclude a detailed discussion of intraoperative monitoring. The interested reader should consult an excellent study by Sala et al.12 For the purposes of our discussion, it is important to mention that intraoperative monitoring tends to cause the surgeon to leave more tumor behind. Recognizing that the most important predictor of survival in pediatric posterior fossa tumors is a gross or near total resection, the neurosurgeon relying on intraoperative monitoring must still accomplish this goal or the tumor will likely progress and the child will die.
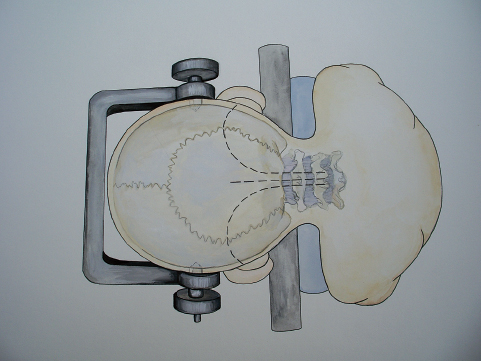
Fig. 25.6 The various skin incisions that can be made in children who will require repeated posterior fossa craniotomies over time, such as children with von Hippel-Lindau disease.
♦ Postoperative Management
Imaging
It has been our practice to inform the parents preoperatively that the child will undergo a postoperative MRI within 48 hours of surgery, and that if this scan demonstrates any resectable residual tumor, the child will be returned to the operating room to remove that remnant. At times these scans may be equivocal, at which point review of the intraoperative video may be useful.
Syndrome of Inappropriate Secretion of Antidiuretic Hormone
All pediatric patients experience a drop in their serum sodium the night after surgery. The exception is the child who develops diabetes insipidus and is volume depleted. Most of the time the sodium level does not drop below 132 mg%. This drop must be closely monitored, as in some children the sodium level drops below 125 mg%, which may precipitate a seizure. Such a seizure may cause bleeding into the fresh wound cavity.
Hydrocephalus
Eighty percent of children with posterior fossa tumors have hydrocephalus at the time of presentation. Most will improve with 24 to 48 hours of intravenous steroids and will not require cerebrospinal fluid (CSF) diversion. In the past, some authors have advocated shunting at presentation and elective tumor resection. St. Rose et al13 have advocated endoscopic third ventriculostomy at presentation. Most of the time, CSF diversion, either temporary or permanent, is not required and the hydrocephalus resolves if the tumor is removed. This may take several days to resolve, however, and the patient must be monitored carefully. In our experience, about one fourth of children ultimately require a shunt for unremitting hydrocephalus.
Swallowing
In instances such as the dorsally exophytic brainstem tumors or the cerebellopontine angle ependymomas, in which the tumor invades the inferior floor of the fourth ventricle or involves the lower cranial nerves, the children are at risk for aspiration pneumonitis in the acute postoperative period. In these instances, it has been our practice to keep the children intubated and sedated overnight following surgery. On the day after surgery, if the child is wide awake, the ENT team is present in the intensive care unit and inspects the vocal cords and pharyngeal motility by fiberoptic endoscopy at the time of extubation. Children with an abnormal examination are maintained on nasogastric feeds and receive nothing by mouth until a formal swallowing study can be performed. For those with vocal cord paralysis or insensate pharynges, we have been quick to recommend tracheostomy and gastrostomy. This routine has prevented all but one death in affected children. That death occurred in a child who was ambulatory following resection of a dorsally exophytic brainstem tumor arising at the obex. She had a tracheostomy and gastrostomy and was in rehabilitation when her parents took her home for a weekend pass. She convinced them to let her drink a soft drink, at which time she aspirated, promptly arrested, and died.
Posterior Fossa Syndrome
One of the earlier reports of posterior fossa syndrome was by Wisoff and Epstein.14 It is a delayed syndrome that occurs 12 to 72 hours following resection of a posterior fossa tumor. The syndrome appears to be a severe dyspraxia in a child who initially awakens from surgery speaking and moving well, but then becomes mute, dystonic, is irritable, and has cognitive impairment. Many have trouble swallowing and have trouble with visual pursuit. The cause of the syndrome is poorly understood. Postoperative imaging is rarely helpful. Some authors have theorized that the syndrome develops from ischemia or edema of the deep cerebellar nuclei. Our current theory, given that it seems to occur most commonly after resection of large midline tumors, is that it is due to sudden decompression of the stretched cerebellar peduncles. The syndrome has been said to occur in 5 to 24% of children following posterior fossa tumor resection. Earlier authors felt that the syndrome always resolved within 6 months or a year of surgery. More recent studies have demonstrated that all of these children have long-term cognitive impairments and emotional problems.15 The posterior fossa syndrome is now recognized as the most common cause of long-term impairment following posterior fossa tumor surgery in children.
♦ Conclusion
Removal of a posterior fossa tumor in a child is one of the most rewarding operations a neurosurgeon can perform. The children present deathly ill, and generally are discharged in good condition. For those with a JPA, the children are cured and can lead a normal life. Even for those with medulloblastoma or ependymoma, major advances have occurred in therapy over the last decade such that 5-year progression-free survival has improved from 35% to 75%. Many of the cognitive impairments seen a decade ago can now be avoided, so that the survivors have a decent quality of life.
References
1. Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst 1999;91:1382–1390 PubMed
2. Schoenberg BS, Schoenberg DG, Christine BW, Gomez MR. The epidemiology of primary intracranial neoplasms of childhood. A population study. Mayo Clin Proc 1976;51:51–56 PubMed
3. Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery 1996;38:265–271 PubMed
4. Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol 2004;22:3156–3162 PubMed
5. Desai KI, Nadkarni TD, Muzumdar DP, Goel A. Prognostic factors for cerebellar astrocytomas in children: a study of 102 cases. Pediatr Neurosurg 2001;35:311–317 PubMed
6. Raimondi AJ. Pediatric Neurosurgery: Theoretical Principles—Art of Surgical Technique, 2nd ed. New York: Springer, 1998
7. Keating RF, Goodrich JT, Packer RJ, Tumors of the Pediatric Central Nervous System. New York: Thieme, 2001
8. Albright AL, Pollack IF, Adelson PD. Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999
9. McLone DG. Pediatric Neurosurgery: Surgery of the Developing Nervous System, 4th ed. Philadelphia: WB Saunders, 2001
10. Albright L. Posterior fossa tumors. Neurosurg Clin N Am 1992;3:881– 891 PubMed
11. Vezina G, Booth TN. Neuroradiology. In: Keating RF, Goodrich JT, Packer RJ, eds. Tumors of the Pediatric Central Nervous System. New York: Thieme, 2001
12. Sala F, Krzan MJ, Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst 2002; 18:264–287 PubMed
13. Sainte-Rose C, Cinalli G, Roux FE, et al. Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 2001;95:791–797 PubMed
14. Wisoff JH, Epstein FJ. Pseudobulbar palsy after posterior fossa operation in children. Neurosurgery 1984;15:707–709 PubMed
15. Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg 2003;39:179–183 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








