Chapter 73 Surgical Management of Posterior Communicating, Anterior Choroidal, Carotid Bifurcation Aneurysms
Approximately 25% of all intracranial aneurysms are PComA aneurysms, making them the second most common overall after anterior communicating artery aneurysms. ICA bifurcation aneurysms account for about 4% to 15% of all intracranial aneurysms; those at the AChA are even more uncommon and comprise less than 5% of such aneurysms.1,2 Although the incidence of intracranial aneurysms in the pediatric population is relatively low, the ICA termination is the most common location of intracranial aneurysms in this patient group.
The first described aneurysm clipping was of a PComA aneurysm by Dandy in 1938 at the Johns Hopkins Hospital. The patient presented with a complete third cranial nerve palsy and periorbital headaches. Dandy described complete obliteration of the aneurysm neck and preservation of the parent vessel with clip application. The patient recovered uneventfully with complete resolution of the third nerve palsy 7 months later.3,4
Embryology of the ICA
Fetal Posterior Cerebral Artery
The caudal division of the ICA continues to grow into the posterior cerebral artery (PCA) by 8 weeks, and the PComA regresses as the vertebrobasilar system develops. The caudal divisions also supply the stems of the PCAs. By 8 weeks, the PCAs may be identified as the posterior communication of the PComAs. If the embryonic PComA fails to regress, the dominant blood supply to the occipital lobes comes from the ICA via the fetal PCA instead of the vertebrobasilar system.5 This occurs in approximately 20% to 30% of cases. A fetal PCA is defined as a variant of the PComA with the same caliber as the P2 segment of the PCA and is coupled with a hypoplastic P1 segment. In this configuration of the circle of Willis, the primary supply to the PCA territory is derived from the fetal PCA; therefore, clipping or coiling of PComA aneurysms must be achieved without jeopardizing flow to this artery.
Diagnosis, Preoperative Planning, and Patient Selection
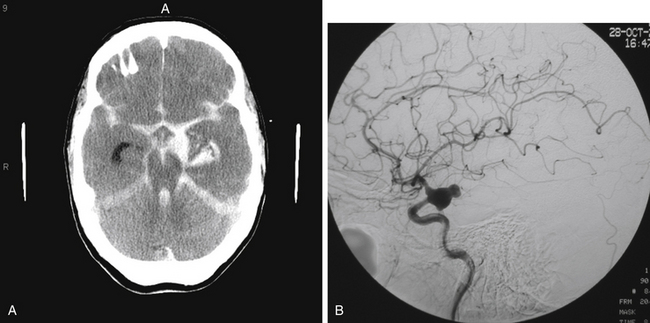
The most common presentation of PComA, AChA, and ICA bifurcation aneurysms is SAH (Fig. 73-1A). While those presenting with SAH are considered for treatment on an emergency basis, preferably within 24 hours of presentation, to minimize the risk of rebleeding, those patients who are found to have symptomatic, unruptured aneurysms should also be considered for urgent treatment. The decision of whether to treat incidental unruptured aneurysms entails consideration of aneurysm- and patient-related factors, such as family history and prior history of aneurysmal SAH. Similarly, the complex choice of either microsurgical or endovascular therapy for any ruptured or unruptured aneurysm rests on multiple factors, such as the patient’s age and medical condition; aneurysm size, location, and morphology; and operator experience. As greater understanding of the natural history continues, technological advances evolve, centralization of care to specialized centers with multidisciplinary capabilities expands, and refined microsurgical and endovascular techniques develop, an aneurysm-specific treatment strategy may be optimally tailored to the individual patient.
Presentation with symptoms caused by mass effect or hemorrhage other than SAH is also possible for these aneurysms. An enlarging unruptured or ruptured PComA aneurysm may also present with a partial or complete, non-pupil–sparing, third nerve palsy. The pupil dilates due to mechanical compression of the parasympathetic fibers that course on the outer surface of the oculomotor nerve. Since relief of the compressive effect caused by the aneurysm is readily accomplished with clipping, improvement in diplopia and ptosis is more often associated with clipping but remains possible with coiling. In univariate analysis,6 the only variable that showed significant association with complete improvement of oculomotor nerve function following microsurgical clipping was severity of the third nerve palsy at admission. In this report, all patients with a partial third nerve palsy regained full oculomotor nerve function. Only 40% of patients with complete third nerve palsy demonstrated complete recovery. Furthermore, there is no statistically significant association between early surgery and improvement of the third nerve function.6 Recovery of third nerve function after coiling has also been documented in case reports.3
Patients presenting with ruptured aneurysms at the PComA (Fig. 73-1B) and AChA (Fig. 73-2) locations may have, in addition to SAH, an associated temporal intracerebral hemorrhage (ICH) or an acute subdural hematoma for which evacuation may be accomplished, along with craniotomy for aneurysm clipping. Ruptured AChA aneurysms often are associated with intraventricular hemorrhage (IVH) and hydrocephalus. Occasionally, a ruptured ICA termination aneurysm manifests with a basal ganglia ICH, with or without IVH.
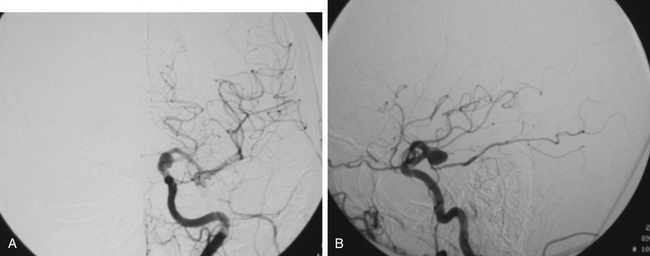
FIGURE 73-2 Left ICA injection on a DSA with an AChA aneurysm: anterior–posterior (A) and lateral (B) views.
In addition, presentation with ischemic symptoms or stroke due to emboli from the aneurysm occurs in about 3.3% of patients. Although more frequently associated with anterior circulation aneurysms, ischemic events in the distal vascular territory attributable to the aneurysm can arise from aneurysms of all sizes, even small aneurysms.7
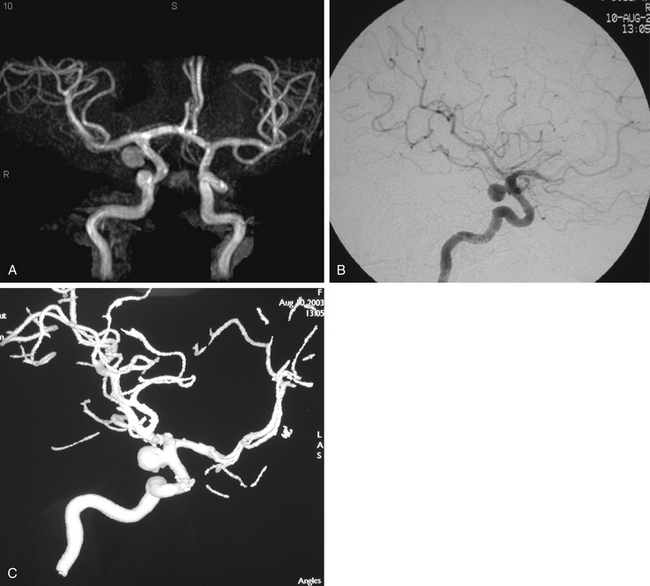
In patients presenting acutely with suspected SAH, computed tomography (CT) scanning remains the initial imaging modality for diagnosis. If the clinical suspicion remains high despite the absence of SAH on head CT, then lumbar puncture is warranted due to its greater sensitivity in detecting SAH. Computed tomography angiography (CTA) has gained increasing utility for aneurysm diagnosis and preoperative planning. Due to the wide availability of magnetic resonance angiography (MRA) and CTA, these noninvasive imaging studies are often the initial screening modality that establishes the diagnosis of an incidentally discovered, unruptured aneurysm (Fig. 73-3A).
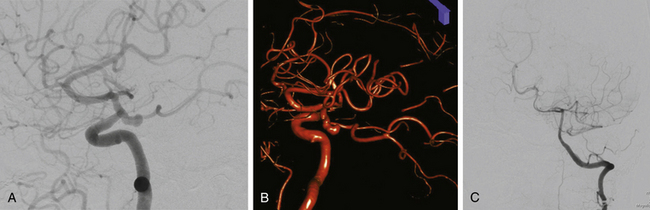
The gold standard for diagnosis and preoperative planning remains the digital subtraction angiogram (DSA) (Fig. 73-3B), as this modality allows selective arterial injections, rotational views, three-dimensional (3D) reconstructions (Fig. 73-3C), and cross-compression testing, if necessary. When assessing the vasculature during the preoperative planning of PComA aneurysm treatment, a fetal PCA (Fig. 73-4A and B) is defined by absence of filling of the PCA or lack of visualization of the P1 segment with the vertebral artery injection (Fig. 73-4C).
In preoperative planning for ICA bifurcation aneurysms, the absence or presence of cross-filling of the ipsilateral ACA across the anterior communicating artery from the contralateral A1 is important to assess with a cross-compression test during the diagnostic angiogram. This maneuver entails compression of the ipsilateral ICA during contralateral ICA injection to determine whether the ipsilateral ACA territory can be supplied by the contralateral ICA. The presence of cross-filling implies that compromise of the ipsilateral A1 during clip reconstruction of ICA bifurcation aneurysms may be compensated for by filling of the ipsilateral A2 via the contralateral A1 across the anterior communicating artery, rather than via the ipsilateral A1.
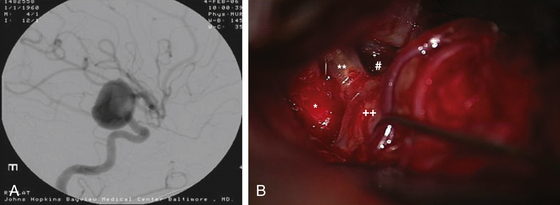
Anterior circulation aneurysms located at the posterior communicating segment (Fig. 73-5), anterior choroidal segment, and terminal ICA bifurcation are typically favorable for microsurgical clipping. Posterior communicating and anterior choroidal aneurysms are carotid wall aneurysms that frequently include the origin of the branch artery at the aneurysm base (Fig. 73-6A). Meticulous identification of the branch artery adjacent to the proximal neck of the aneurysm allows for clip application across the neck to be accomplished with simultaneous preservation of the origin of the branch artery and minimal brain retraction (Fig. 73-6B to E).
The ICA termination aneurysms that may be relatively more favorable for endovascular therapy than microsurgical clipping are those with heavily calcified walls or a primary posterior projection.8 CT or CTA (Fig. 73-7) may be useful in treatment planning to determine the presence of calcification in the aneurysm walls. Using clip blades to gather the neck of a thick-walled aneurysm in the presence of severe atherosclerosis and calcification may risk hemodynamically significant stenosis of the proximal A1 or M1 segment at its origin from the terminal carotid bifurcation. A strategy of placing the clips more distally on the neck to avoid constriction of the origins of the A1 and M1 segments may be employed. Endovascular therapy may be preferable for those ICA termination aneurysms that project posteriorly. Since the perforators originate from the distal ICA at its posterior wall, clipping of posteriorly projecting ICA termination aneurysm may be treacherous due to poor visualization of the perforating arteries.
Anatomic and Clinical Considerations
Posterior Communicating Segment
The communicating segment of the ICA courses between the optic and the oculomotor nerves to the anterior perforated substance at the medial section of the sylvian fissure.2 Angiographically, this segment extends from the origin of the PComA to the origin of the AChA. The PComA arises from the posteromedial or posterior surface of the ICA; courses medially and inferiorly, superior and medial to the oculomotor nerve, through the membrane of Liliequist; and joins the PCA at the junction of the P1 and P2 segments. Multiple, variable perforating arteries arise from the PComA, namely, the anterior thalamic perforators, which are at risk of compromise during aneurysm clipping.
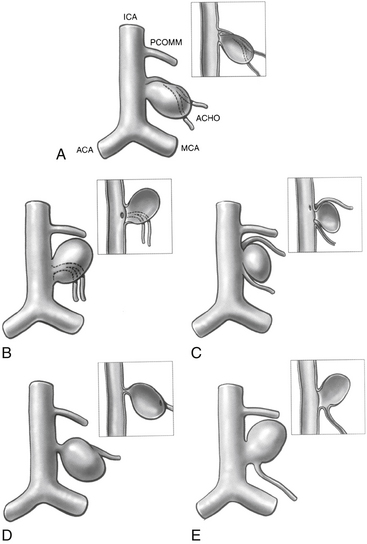
PComA aneurysms are most commonly ICA aneurysms that arise from the ICA and incorporate the origin of the PComA into its neck, rather than arising directly from the PComA. PComA aneurysms are considered relatively straightforward aneurysms for clipping, because their location is very proximal on the supraclinoid ICA. They comprise approximately 25% of all ruptured aneurysms.9 The direction of the dome projection has significant implications for presentation and microsurgical treatment. Yasargil has described a classification system based on the direction of the dome.10 When the dome projects anterolaterally, the origin of the PComA is likely to be obscured from view by the aneurysm. In addition, the aneurysm dome may adhere to the anterior clinoid process. This situation requires cautious dissection to mobilize the dome and visualize the origin of the PComA during clip placement. A superolateral dome is occasionally encountered in which the dome points toward the medial sphenoid ridge. Rupture of these aneurysms is likely associated with presentation with a subdural hematoma. Rare cases of a contralateral frontal ICH from PComA aneurysm rupture have been reported in the literature.11 Aneurysms with a posterolateral superior projection point into the medial temporal lobe and are often associated with intraparenchymal hemorrhage or even hemorrhage into the temporal horn of the lateral ventricle. PComA aneurysms with a posterolateral dome projection may present with a dilated pupil and oculomotor paresis due to compression of the third cranial nerve. A posterolateral inferior fundus may penetrate the membrane of Liliequist with projection into the interpeduncular fossa. Comprising approximately 5% of all PComA aneurysms, the variant of a “true PComA aneurysm” refers to the configuration in which the neck of the aneurysm originates entirely from the PComA.9,12–14 Essential for operative planning is review of the angiogram to determine whether the PCA is supplied by the posterior circulation and fills from the vertebral artery injection or is fetal in configuration and fills from the ICA injection. In the 4% to 25%3,9,15,16 of patients who have a fetal PCA, the P1 segment of PCA is hypoplastic, and the PCA arises directly from the PComA. PComA aneurysms associated with a fetal PCA must be reconstructed either with clips or with coils in a manner that preserves the patency of the ICA and fetal PCA vessels. In contrast, a diminutive PComA may be sacrificed if necessary.
The origin of the PComA is usually found proximal and medial to the proximal neck of the aneurysm. PComA aneurysms typically project laterally and posteriorly; they rarely project medially.2 The arachnoid of the sylvian fissure should be divided appropriately to reveal the medial border of the temporal lobe and to appreciate the PComA origin and the course and number of AChA vessels. The relationship between the dome of the aneurysm and the medial temporal lobe should not be disturbed before the arachnoid has been dissected adequately and proximal control of the ICA has been achieved. The dome of the aneurysm may adhere to the edge of the tentorium. If the dome is attached to the medial temporal lobe, temporal lobe retractors should be avoided or used cautiously. The two most common causes of intraoperative rupture are premature medial retraction of the supraclinoid ICA and retraction of the temporal lobe while the aneurysm dome remains adherent to the temporal lobe. In general, temporal lobe retraction should be avoided in ruptured cases.
In rare instances, a PComA aneurysm may be partially obscured by the anterior clinoid process.17,18 In this situation, adequate proximal control of the ICA and visualization of the proximal neck of the aneurysm may necessitate partial removal of the anterior clinoid process, resection of the anterior petroclinoid fold, or exposure of the ICA in the neck. A predictor of the need for clinoidectomy is a short distance between the tip of the anterior clinoid process and the proximal neck of the aneurysm assessed using CTA.
Adequate dissection of the sylvian fissure facilitates identification and preservation of the AChA. This artery may travel medial to the aneurysm and adhere to the dome of the aneurysm at its cisternal segment, making it necessary to separate the vessel from the distal neck of the aneurysm. The AChA should be preserved in all instances. The AChA supplies the optic chiasm, optic tract, lateral geniculate nucleus, uncus and amygdala, middle third of the cerebral peduncle, caudate, globus pallidus, genu and posterior limb of internal capsule, anterior hippocampus and dentate gyrus, fornix, pulvinar, and choroid plexus.19
Exposure of the contralateral PComA and choroidal segment can be accomplished either through the interoptic space or through the contralateral opticocarotid space. Aneurysms at these locations are the most difficult aneurysms to expose via a contralateral approach. Boundaries of the triangular contralateral opticocarotid space are the lateral aspect of the contralateral optic nerve, chiasm, and tract; the medial aspect of the contralateral ICA; and the inferior aspect of the precommunicating segment of the contralateral ACA.20 Since these aneurysms frequently project posterolaterally, the neck region and origins of the PComA and AChA may be partially obscured by the contralateral ICA and optic apparatus. Optic chiasm position can interfere with visualization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree