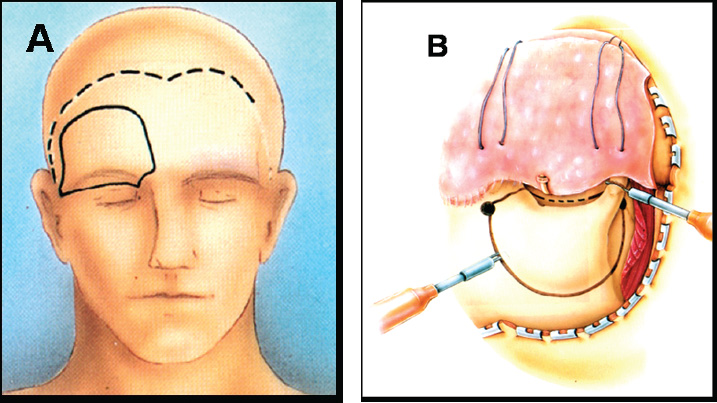
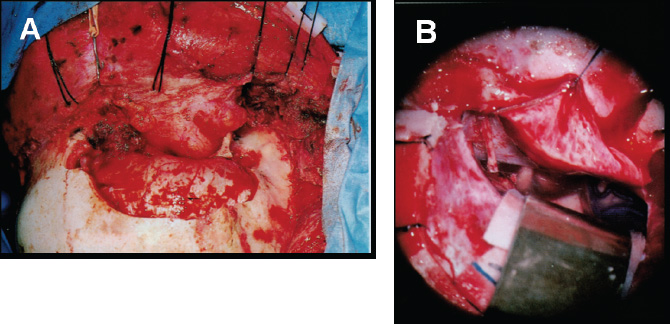
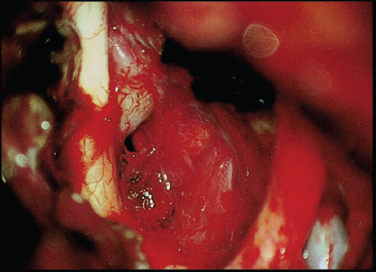
7 Surgical Treatment of Meningiomas The surgeon approaching tumors in the parasellar region must contend with a highly congested neurovascular anatomy surrounding the pituitary gland and stalk. In particular, the optic chiasm and nerves, carotid artery and branches, and upper cranial nerves merit meticulous attention, especially when meningiomas of unforgiving consistency are handled. Accumulated experience with meningiomas and other parasellar tumors has established surgery as a technically feasible and clinically durable method of managing them in patients in whom tumor control by total removal is desired and in whom transsphenoidal access is ill advised. Below, we review the clinical presentation, radiographic features, and surgical treatment principles of meningiomas of the parasellar region, focusing on surgical management of tumors of the tuberculum sellae, diaphragma sellae, cavernous sinus, and anterior clinoid. Such approaches used for meningiomas are generally applicable to other parasellar and sellar tumors. We review the two primary surgical approaches adopted for tumor resection in this region—the supraorbital and the cranio-orbitozygomatic—and discuss the tumors attacked with each technique. Although each tumor type warrants individual consideration, the authors use two primary surgical approaches to the parasellar region: the supraorbital and the cranio-orbitozygomatic (COZ).1 The supraorbital approach minimizes frontal lobe retraction while providing unobstructed access to the floor of the anterior fossa from planum to the sella. The COZ approach affords wide exposure of the entire cavernous sinus and the proximal and distal carotid artery with minimal cerebral retraction. We usually use the supraorbital approach for tuberculum sellae and diaphragma sellae meningiomas. We use the COZ frequently for cavernous sinus and clinoidal meningiomas, which often encase the carotid artery and require arterial control. Below, we review the technical features of each approach and the tumor types commonly resected with their application. We perform this approach based on that originally described by Jane et al.2 For the supraorbital approach, the patient is placed supine and the head and trunk elevated 20 degrees so the head is above the heart. The head is moderately extended to allow the frontal lobes to fall backward and kept straight to facilitate anatomic orientation. We monitor somatosensory evoked and brainstem auditory evoked potentials. For patients with small to medium-sized tumors, a spinal drain is inserted so that spinal fluid may be released in a controlled fashion for brain relaxation. The scalp incision is begun 1 cm anterior to the tragus and is continued in curvilinear fashion behind the hairline to the level of the superior temporal line on the opposite side (Fig. 7.1A). The superficial temporal artery is behind the incision, and the facial nerve branches are in front of the incision. A large pericranial flap based on the supraorbital and frontal vessels beginning well behind the skin incision and extending from one superior temporal line to the other is raised and reflected over the scalp flap anteriorly and dissected offthe superior and lateral orbital walls. The supraorbital nerve is freed from its notch or freed with a high-speed drill from its foramen. The superior aspect of the temporalis muscle is the only part that needs to be mobilized. To do this, a subfascial dissection of the temporalis fascia is performed by incising both layers of temporalis fascia to expose the temporalis muscle, beginning at the keyhole and proceeding behind the course of the facial nerve to approximately the level of the sylvian fissure. The fascial layers are reflected forward, and the temporalis muscle, dissected free from its overlying fascia, is released from its insertion at the superior temporal line and along the superior and lateral orbit and reflected inferiorly. The junction of the zygomatic, sphenoidal, and frontal bones is thus exposed. The bone flap is usually a unilateral supraorbital bone flap, although large tumors may be approached with a bifrontal supraorbital flap. The unilateral flap begins with placement of a burr hole at the anatomic keyhole ~1 cm behind the frontozygomatic suture (Fig. 7.1B). When drilled correctly, the superior half exposes the frontal lobe dura, and the lower half exposes the periorbita, with the orbital roof in the center of the burr hole separating the frontal lobe and orbit. A more posterior burr hole on the superior temporal line may also be made. The frontal aspect of the keyhole burr hole and the posterior superior temporal burr hole are connected with the craniotome, and the craniotomy continues into the frontal bone ~4 cm above the superior orbital rim and is curved medial to the supraorbital notch to avoid the frontal sinus. An additional cut is made in the lateral orbital rim and continued to the keyhole. Finally, the flap is completed with a superior orbital roof osteotomy by placing a chisel straddling the lateral aspect of the superior orbit exposed in the keyhole and completing the osteotomy. If the frontal sinus is entered during the exposure, the sinus is exenterated and its posterior wall removed. A small piece of fat or temporalis muscle is used to pack the sinus and thereby obliterate the frontonasal duct. In Fig. 7.2A, the craniotomy flap has been removed, but the dura is intact. After tack-up stitches are placed, the dura is opened transversely and reflected over the orbit. In a bilateral approach, the sagittal sinus is ligated and the falx cut anteriorly. Because the superior orbital rim is removed, frontal lobe retraction is minimal (Fig. 7.2B). The frontal lobe is gently elevated and the olfactory nerve preserved by dissecting it offthe inferior frontal lobe; a drop of fibrin glue can help decrease the risk for avulsion. Tumor feeders in tuberculum sellae tumors—typically arising inferiorly from the internal maxillary or posterior ethmoidal arteries—are cauterized medially, avoiding optic nerve injury laterally. The tumor is then debulked with a combination of ultrasonic aspiration and bipolar cautery. Once the tumor is debulked, the remainder of the tumor is carefully dissected from the optic nerves, often starting near the chiasm and carefully preserving arterial supply to the optic apparatus. Although the tumor often appears adherent to the nerve, a plane of dissection can usually be established. Tuberculum sellae tumors usually extend into at least one optic canal, so one or both may need opening; cavernous sinus extension is managed as explained previously in this chapter. The ophthalmic artery and its branches should be preserved when tumor inside the optic canal is removed. The carotid is dissected from the tumor, as are its branches, with careful attention paid to the A1 segments because they may be stretched and susceptible to tearing. The pituitary stalk is distinct in its color and vascular arborization (Fig. 7.3). Tuberculum sellae meningiomas usually displace the stalk posteriorly and rarely will engulf the stalk, requiring careful microdissection. Hypophyseal branches offthe carotid are spared. The involved dura of the tuberculum and planum is resected and any hyperostotic bone drilled with a high-speed diamond burr. Paranasal sinus entry is handled by exenterating the sinus mucosa and packing the spaces with fat. A piece of free pericranium or fascia lata is then placed intradurally and secured to the native dura over the resected dura, and after the dural closure, the pericranial flap is turned over the orbit and frontal bone and spread over any defect in the anterior fossa floor. These tumors, originating in front of the sella and arising from the tuberculum, chiasmatic sulcus, and limbus sphenoidale, account for 5 to 10% of meningiomas in the series of Cushing and Eisenhardt.3 They often invade the floor of the anterior fossa, causing hyperostosis commonly in front of the sella (Fig. 7.4A). As they enlarge, they displace the optic nerves laterally and chiasm superiorly (Fig. 7.4B). They also impinge on the pituitary stalk posteriorly, as well as the basilar artery, and can extend to the interpeduncular space. Although preoperative pituitary dysfunction is uncommon in these tumors, a preoperative endocrine evaluation is still recommended. The blood supply is typically inferior from the posterior ethmoidal arteries, and meticulous attention must be paid to the anterior cerebral artery complex, which, depending on the size, may be encased or displaced superiorly. Careful attention must be paid to small medial lenticulostriates. The relationship of these tumors to the visual apparatus confers their dominant clinical presentation. The classic clinical manifestation, the chiasmal syndrome, denotes a primary optic atrophy with a bitemporal field defect.4 Visual field defects are typically incongruous and asymmetric. In the senior author’s most recent series, quadrantanopsia and a unilateral temporal defect were the most common visual findings.5 More than 50% of patients reported failing vision in one eye. A markedly asymmetric visual field examination has been found to be highly indicative of optic canal involvement, which has specific surgical implications.6 A full visual field and acuity examination is warranted before surgery. We favor the unilateral or bilateral supraorbital approach as originally described by Jane et al,2 as previously stated. The main principles of the approach as it pertains to tuberculum sellae tumors are the low frontobasal exposure, which facilitates access to the tumor without undue frontal retraction and yet access to both optic canals. Removing the supraorbital rim as part of the frontal craniotomy flap affords access to the base of the anterior fossa without frontal lobe retraction; further relaxation is achieved by spinal drainage. When the tumor is encountered, its basal posterior ethmoidal arterial feeders are coagulated to devascularize the tumor, after which central debulking may be undertaken by ultrasonic aspiration or other means. The optic chiasm is usually displaced superiorly and the optic nerves superiorly and laterally. Should the nerves be engulfed in tumor, dissection should begin at the chiasm and proceed proximally. The arterial supply to the optic chiasm and nerves is preserved. All associated dura is resected, and any hyperostotic bone at the tuberculum and planum is drilled away with a high-speed diamond burr. Decompression of the visual apparatus is not complete until the optic canals are unroofed; in a recent series, 67% of tuberculum sellae tumors were associated with tumor extension into one (40%) or both (60%) optic canals.4 Moreover, tumor left inside the optic canal may be the source of recurrence or of mitigated visual improvement after surgery.7 One optic canal or both are exposed and unroofed with a high-speed drill under constant irrigation to avoid thermal injury to the optic nerve. The falciform ligament and optic sheath are opened sharply. Optic nerve decompression should proceed as far anteriorly toward the globe as is needed to ensure that all optic extension of the tumor has been extirpated. Although the clinical presentation may suggest extension of the tumor into only one optic canal, one should be prepared to decompress both canals based on intraoperative findings. Even the slightest suggestion of canalicular extension should prompt at minimum opening of the falciform ligament. Visual improvement was noted in 74% of patients who presented with visual deterioration in the senior author’s series. Pituitary dysfunction is rare after surgery, and if present, it is usually transient in the early postoperative period. Although the entity was first reported in 1954,8 diaphragma sellae meningiomas were not formally classified until more recently9; they are probably considered along the spectrum of tuberculum tumors in most series. Diaphragma sellae meningiomas may be considered distinct clinical entities separable into three types according to their relationship to the pituitary stalk and their specific diaphragmatic origins (Fig. 7.5). Each subtype is also associated with particular clinical symptoms related to proximity to the optic apparatus, pituitary, hypothalamus, and limbic system. Surgically, types A and B are approached similarly to tuberculum tumors, whereas type C tumors may be approached transsphenoidally. Type A tumors originate from the upper leaf of the diaphragma sellae anterior to the pituitary stalk. In this way, these tumors resemble tuberculum sellae tumors and do present with visual field disturbances, but they are more likely than tuberculum tumors to present with manifestations of pituitary compromise, such as diabetes insipidus. Headache is also a common presenting symptom. On imaging, the tumors are supradiaphragmatic and may be difficult to differentiate from tuberculum sellae tumors. Type B tumors also originate from the upper leaf of the diaphragma and are supradiaphragmatic, but unlike type A tumors, they are located posterior to the stalk and feature a greater incidence of pituitary disturbance preoperatively. Common presenting symptoms include headache, unilateral and bilateral visual disturbances in 50% of patients, and pituitary dysfunction in 38% of patients. These are also supradiaphragmatic on imaging, with a normal sella. Unlike type A and B tumors, type C tumors arise from the inferior leaf of the diaphragma and are thus infradiaphragmatic. Symptoms are similar to those produced by nonfunctioning pituitary adenomas. Half of patients presented with bitemporal hemianopsia, and 40% presented with hypopituitarism. Distinct from types A and B tumors, enlarged sellar and intrasellar tumors are demonstrated on imaging. Diagnostically, it may be difficult to distinguish types A and B tumors from tuberculum sellae meningiomas, and type C tumors from pituitary adenomas, although the latter distinction may be aided by the more avid enhancement in type C tumors and smaller sella in comparison with pituitary adenomas.10 In the senior author’s original description, patients with type A tumors had excellent outcomes; those with type B or C tumors had higher rates of transient pituitary dysfunction postoperatively. Although transsphenoidal approaches may be attempted in type C tumors, the supraorbital approach offers excellent access to types A and B tumors. The COZ approach adds an anterolateral skull base approach to the supraorbital approach, rendering it ideal for accessing tumors that may engulf or displace structures superiorly and laterally in the parasellar region. A lumbar drain is placed for cerebrospinal fluid (CSF) drainage, and the patient is positioned supine with the upper body slightly elevated and the head rotated 30 degrees to the opposite side. Leads to monitor somatosensory evoked potentials, brainstem auditory evoked potentials, and cranial nerves V and VII are placed at this time, with those to monitor nerves III, IV, and VI placed later. The ipsilateral part of the neck is prepared should more proximal carotid control be required, and the abdomen is prepared for fat graft harvest. The skin incision for the COZ approach starts at the zygomatic root and is carried behind the hairline toward the contralateral superior temporal line (Fig. 7.6A). The superficial temporal artery is identified and carefully protected, and a large pericranial flap is raised by undermining the scalp posterior to the incision and dissecting sharply against the scalp flap anteriorly. A subfascial dissection of the temporalis fascia is performed to preserve the frontal branches of the facial nerve. The zygomatic arch and superior and lateral orbital margins are exposed by subperiosteal dissection, after which the zygoma is divided at either end and displaced inferiorly on its masseteric pedicle. The temporalis muscle is then elevated in subperiosteal fashion, beginning low on the temporal squama and proceeding superiorly to detach the muscle at the superior temporal line. The entire temporalis muscle is then reflected inferiorly with the freed zygoma. The superior and lateral orbital rims are dissected free from the periorbita, with the supraorbital nerve and vessels preserved (Fig. 7.6B). A burr hole is placed in the keyhole to gain simultaneous entrance into the cranium and orbit. Burr holes are then placed anteriorly and posteriorly, adjacent to the temporal floor. A cut is made from the medial aspect of the lateral orbital wall to its lateral aspect and is continued to the keyhole. The keyhole is then connected to the posterior burr hole by cutting through the temporal fossa. A cut starting at this burr hole is brought superiorly to the frontal bone, then anteriorly through the supraorbital rim, with care taken to protect the orbital contents during any cuts involving the bony orbit. Care must be taken to ensure that the posterior wall of the frontal sinus is cut if the sinus has been entered. A cut is made from the first burr hole through the orbit, again with care taken to protect the orbital contents. A notched osteotome is used to incise the orbital roof from the second burr hole toward the nasion, while the orbital contents are protected during this cut. The bone flap is now elevated. Remaining portions of the orbital roof, lateral orbital wall, and sphenoid wing can be removed with the craniotome for later reconstruction. With the orbit now exposed, electromyographic electrodes may be directly placed into the superior oblique, superior rectus, and lateral rectus muscles to monitor cranial nerves III, IV, and VI. Proximal control of the carotid artery is the next objective. The middle fossa dura is elevated in a posterior-to-anterior direction. The greater superficial petrosal nerve (GSPN) emerges from the facial hiatus and should be dissected free of the dura. Traction of the GSPN is avoided to alleviate transmission to the geniculate ganglion, which can lead to facial palsy. The middle meningeal artery is identified, thoroughly electrocoagulated, and divided. Continued dural elevation reveals nerve V3 and the foramen ovale. The apices of Glasscock’s triangle are now exposed: the facial hiatus, the anterior aspect of the foramen ovale, and the intersection of the GSPN and the lateral aspect of V3. This triangle overlies the carotid artery, and drilling here with a diamond bit and constant irrigation exposes the carotid artery. This may be sufficient for proximal control of the artery or to allow drilling posterolaterally from the known location of the artery. Proximal control may be obtained by sufficient exposure for placement of a temporary clip on the petrous carotid artery, if necessary. Alternatively, a Fogarty catheter may be inserted into the carotid canal. Should vascular control be required, the catheter balloon can be inflated to occlude the carotid artery in the carotid canal.11 Medial exposure of the cavernous sinus and exposure of the paraclinoid carotid artery are obtained by drilling out the remainder of the orbital roof, the superior orbital fissure, the anterior clinoid process, and the optic strut. Drilling adjacent to the orbital apex and optic canal mandates a diamond burr and copious irrigation to dissipate the heat of drilling. The anterior clinoid process is cored out with the drill and then disarticulated by drilling out the optic strut. The clinoid is subperiosteally dissected and resected. The superior orbital fissure is opened by drilling along the lesser sphenoid wing. This procedure exposes the subclinoid portion of the carotid artery, which is both extradural and extracavernous, and provides distal control of the carotid artery. Entrance into the cavernous sinus has been described in relationship to the intervals between the neurovascular structures of the cavernous sinus. These intervals have been annotated as 10 triangles distributed among the parasellar, middle fossa, and paraclival locations.12 The actual approach taken depends on the anatomy of the lesion in relationship to the cavernous sinus structures and must be individualized for each patient. In general, there are two approaches for entry into the cavernous sinus: a superior approach and a lateral approach. The superior approach is particularly suited to those lesions adjacent to the anterior loop of the carotid artery, and those that are superior and/or medial to the cavernous carotid artery. The lateral approach lends itself well to exposing those lesions lateral and/or inferior to the carotid artery and those that are posteriorly located within the cavernous sinus. Frequently, these approaches are combined for lesions widely involving the sinus. After the superior surface of the cavernous sinus has been exposed (Fig. 7.7A), the dura overlying the optic nerve is divided over the length of the optic canal to free the optic nerve. The distal carotid ring is now divided. The dura is then incised toward the oculomotor nerve, providing initial entry into the cavernous sinus. Exposure can be increased by dissecting along the length of the carotid artery. Further exposure can be obtained by subperiosteal dissection of the posterior clinoid process and drilling offthe process, the dorsum sellae, and the superior clivus. These maneuvers allow increased exposure of the posterior fossa. For tumors with medial extension, the planum sphenoidale can be drilled away. This allows exposure of the sphenoid sinus. Dissection and incision of the diaphragma sellae allow visualization of the pituitary gland. Great care must be taken during closure to obliterate any communication between the cavernous sinus and sphenoid sinus to prevent CSF leakage. Lateral entry into the cavernous sinus can be intradural or extradural. Extradural entry begins by incising the dura propria overlying V3. The dura propria is peeled away from the trigeminal branches and ganglion with superiorly directed traction. This will initially expose the third division and lateral ganglion, followed by the second division and most of the remainder of the ganglion. Drilling bone here will also free the trigeminal branches and will, in turn, allow greater mobility of these branches and the ganglion. A mass beginning to enter the posterior fossa can be further exposed by drilling the petrous apex. This drilling also allows greater exposure around and under the trigeminal ganglion. For lesions requiring intradural exposure, intradural entry into the cavernous sinus is achieved through Parkinson’s triangle (Fig. 7.7B). Cranial nerves III and IV are identified over the tentorial edge. An incision beneath the anticipated position of the fourth nerve is fashioned and extended ~8 mm anteriorly and 8 mm inferiorly. The external dural layer is peeled away from the thin inner dural layer in which nerves III, IV, and V are found. The dural flap can be further dissected from the trigeminal ganglion to expose the Meckel cave. Exposure can be increased posteriorly and into the posterior fossa by drilling the petrous apex. The inner dural layer between the fourth nerve and the ophthalmic division can be incised to expose the lateral space of the cavernous sinus, the posterior bend and the horizontal segment of the intracavernous carotid artery, and the lateral cavernous and meningohypophyseal arteries. The abducens nerve is the only cranial nerve coursing inside the cavernous sinus proper, often appearing in fascicles of two to five nerves, and should be carefully located and protected. Frequently, meningiomas necessitate the combination of extradural and intradural cavernous sinus dissection with a combination of superior and lateral entry. The development, refinement, and careful application of skull base approaches, in particular the COZ approach, has made obsolete the notion of the cavernous sinus as a surgical “no man’s land.” Cavernous sinus meningiomas have an estimated incidence of 0.5 per 100,000.13 Meningiomas may involve the cavernous sinus either primarily or secondarily. Those originating from within the cavernous sinus proper may extend to the Meckel cave, medially to the sella, and to the anterior, middle, or infratemporal fossae. Clinoidal, medial sphenoid wing, and petroclival meningiomas may extend to the cavernous sinus secondarily. Patients with tumors in the cavernous sinus may present with symptoms referable to compression or congestion of anatomic structures in or near the cavernous sinus. Proptosis, headache, facial pain or numbness, and disturbances of ocular function or motility (diplopia, ptosis, anisocoria, complete ophthalmoplegia) are common. Tumors can compress the optic nerve, with resultant visual field deficits. Cavernous carotid artery compression may result in ischemic deficits. Less commonly, patients may present with pituitary dysfunction. Physical examination should include a thorough neurologic examination, with particular attention paid to the function of cranial nerves II through VI, including a formal visual field assessment. An endocrine evaluation should be performed because tumors may displace the stalk and gland. Examination of coordination and motor, sensory, and cerebellar functions assists the assessment of any tumor extension into the posterior fossa with brainstem compression. The outcome of any treatment for cavernous sinus meningiomas must be weighed against the natural history of these tumors. Asymptomatic patients or minimally symptomatic patients with cranial nerve involvement may be managed conservatively. Technical advances in skull base surgery have established the cavernous sinus as an approachable space whose contents may be navigated successfully during meningioma surgery. Core principles of these approaches include maximal bone removal for exposure; the avoidance of brain retraction; and control of the carotid artery in its petrous, cavernous, and/or clinoid segments. Two primary surgical approaches to the cavernous sinus are used by the authors: the COZ approach and the zygomatic approach.14 The COZ approach affords wide exposure of the entire cavernous sinus and the proximal and distal carotid artery with minimal cerebral retraction. The zygomatic approach is used for tumors in the posterior cavernous sinus and petrous apex. This approach is more limited, does not offer readily obtainable distal carotid control, and does not expose the medial or superior cavernous sinus as easily as does the COZ approach. During tumor resection, arachnoid planes facilitate tumor removal, but these planes become scarred and obliterated after initial resection or irradiation, emphasizing the importance of extensive resection during an initial operation. If necessary, when no plane is encountered, small remnants of adherent tumor are left on the carotid artery. The same philosophy is true regarding the cranial nerves. As mentioned, once the cavernous sinus is entered, the abducens nerve should be located and preserved. Any cranial nerve that is frankly severed should be directly repaired. If a tension-free direct repair is not possible, an interposition graft should be performed. Injury to the carotid artery can be addressed in several ways. Temporary clipping and direct repair of tears can be performed with 8–0 suture; more severe carotid injury can be treated by vein graft repair. Attempts should be made to remove all affected dura, and any affected dura that cannot be resected should be electrocoagulated, if possible. All involved or hyperostotic underlying bone should be drilled away. Any submucosal tumor spread within the paranasal sinuses should be removed. Sphenoid sinus defects must be repaired with extreme care during the reconstruction. In the senior author’s experience, gross total resection (GTR) in a series of 41 patients was achieved in 76% of patients, with an 11% recurrence rate in cases of GTR. A more recent review of 163 cases operated on by the senior author (OAM) has shown a GTR rate of 44%, with 7% of these patients experiencing recurrence. Stroke is a significant concern during cavernous sinus meningioma surgery, and fortunately reported rates of ischemic complications; in the senior author’s series, 7 of 188 patients (3.7%) experienced ischemic stroke. Cushing and Eisenhardt recognized this entity in their original treatise, noting that there exist meningiomas arising from “the deep or clinoidal third.”15 Although they were historically grouped in discussions of medial sphenoid wing meningiomas, an increasing recognition of clinoidal meningiomas16,17 has culminated in an anatomic description of these meningiomas in three distinct subgroups (types I, II, and III) with specific surgical implications 18,19 (Fig. 7.8). In group I tumors, the tumor originates proximal to the end of the carotid cistern, typically from the undersurface of the anterior clinoid process. In this manner, the tumor may encase the carotid adventitia without an intervening arachnoidal layer. The tumor thus grows along the vessel wall to the carotid bifurcation and beyond, “pushing” a sleeve of arachnoid with it. The absence of an arachnoidal layer between tumor and vessel greatly complicates the dissection. However, the optic chiasm and nerves are invested by the arachnoid of the chiasmatic cistern and should be able to be freed from tumor. Group II tumors originate from the superior or lateral aspect of the anterior clinoid process above the carotid and are invested by cisternal arachnoid. In these cases, a layer of arachnoid separates tumor from vessel from the carotid to the bifurcation and into the sylvian fissure. Microdissection of meningioma from the carotid and its branches is thus rendered feasible by the investing layer of arachnoid, which separates tumor from vascular adventitia. As with group I tumors, the optic apparatus is sheathed in arachnoid and can be dissected from tumor. In group III, the tumor originates on the medial aspect of the anterior clinoid process at the optic foramen. These tumors are usually small because they present early with visual compromise. The arachnoidal layer may be present between tumor and carotid but may be absent between tumor and optic nerve. Clinoidal meningiomas commonly present with initial unilateral visual loss, frequently associated with optic atrophy; the contralateral eye may be affected, depending on chiasmal involvement. Cranial nerve involvement and exophthalmos may occur as the tumor involves the superior orbital fissure and cavernous sinus. Preoperative endocrine disturbance is rare. Preoperative imaging should include fine-cut computed tomograms through the skull base to assess hyperostosis in the region of the anterior clinoid process, optic strut, and superior orbital fissure. Standard magnetic resonance (MR) imaging sequences should be paired with vascular imaging (eg, MR arteriography and MR venography) because the carotid may be narrowed owing to tumor encasement. The COZ approach allows a shorter working distance to the tumor; minimizes brain retraction; and permits subfrontal, transsylvian, and subtemporal routes of attack. The anterior clinoid process should be removed to resect involved bone, intercept middle meningeal artery feeders, and facilitate internal carotid artery exposure. We typically do this extradurally. Once the dura is opened, CSF drainage is accomplished by splitting the sylvian fissure and facilitating partial CSF egress through a lumbar catheter. Tumor dissection should begin with a sylvian fissure split, during which tumor may be encountered. Dissection should continue along the middle cerebral artery to the carotid and to the anterior cerebral artery and Heubner’s artery, respecting the medial and lateral lenticulostriates. Laterally, the posterior communicating and anterior choroidal arteries reside in a separate cisternal compartment, facilitating this dissection. Posteriorly, Liliequist’s membrane is usually intact, facilitating removal from the interpeduncular fossa. The optic nerve may be elevated, depressed, or engulfed in tumor—it is often easiest to find the nerve at the chiasm and dissect toward the optic canal, with care taken to preserve the vascular supply of the nerve. If tumor extends into the optic canal, the falciform ligament should be opened and the optic canal unroofed, followed by optic sheath sectioning. The complete resection of groups I and III tumors depends on the adherence of tumor to the carotid and optic nerve, respectively; an intervening arachnoid layer may be absent. Group II meningiomas in the senior author’s series could be completely resected. Parasellar meningiomas and other tumors of this area are challenging lesions whose proximity to cranial nerves, the carotid artery, the brainstem, and the pituitary gland mandates meticulous microsurgical technique. With the exception of type C diaphragma sellae meningiomas, parasellar tumors are usually managed with such skull base approaches as the COZ or supraorbital technique. A thorough understanding of the patient’s history, physical examination findings, and radiographic findings and the results of other evaluations is crucial. Although pituitary dysfunction is uncommon, preoperative and postoperative endocrine evaluation is nevertheless essential because the gland and stalk are often displaced and manipulated during surgery.
 Surgical Approaches
Surgical Approaches
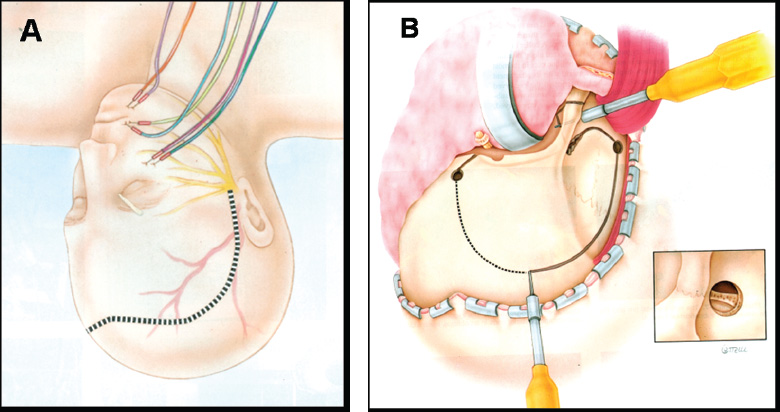
 Supraorbital Approach: Technique
Supraorbital Approach: Technique
 Supraorbital Approach: Applications to Parasellar Tumors
Supraorbital Approach: Applications to Parasellar Tumors
Tuberculum Sellae Meningiomas
Specific Surgical Principles
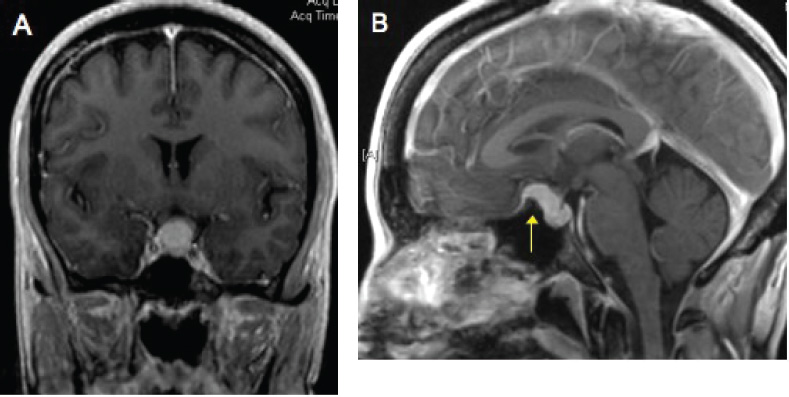
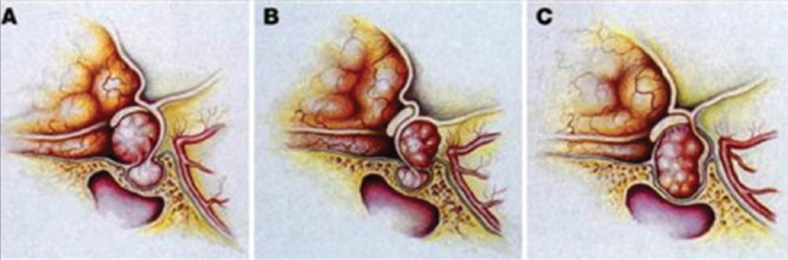
Diaphragma Sellae Meningiomas
 Cranio-orbitozygomatic Approach: Technique
Cranio-orbitozygomatic Approach: Technique
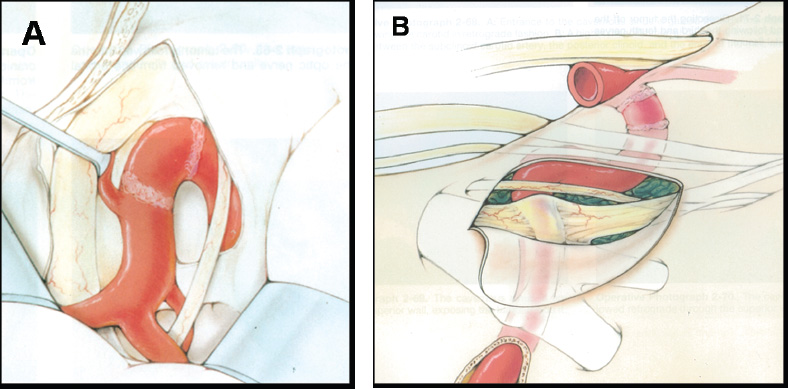
Superior Entry
Lateral Entry
 Cranio-orbitozygomatic Approach: Applications to Parasellar Tumors
Cranio-orbitozygomatic Approach: Applications to Parasellar Tumors
Cavernous Sinus Meningiomas
Specific Surgical Principles
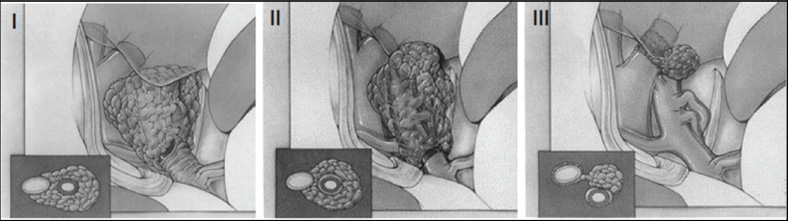
Clinoidal Meningiomas
Specific Surgical Principles
 Conclusion
Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


