Chorea is a hyperkinetic syndrome characterized by brief, abrupt involuntary movements resulting from a continuous flow of random muscle contractions. The pattern of movement may sometimes appear playful, conveying a feeling of restlessness to the observer. When choreic movements are more severe, assuming a flinging, sometimes violent, character, they are called ballism. Regardless of its etiology, overall chorea has the same motor features, although the muscle tone varies depending on the underlying etiology (1). As several basal ganglia circuits are often involved in conditions associated with chorea, nonmotor features such as subcortical cognitive decline, obsessions, compulsions, attention deficit, and others are often present in choreic syndromes. There are genetic causes of chorea, described in other chapters of this book, and nongenetic causes of chorea, listed in Table 20.1. The latter include vascular choreas, autoimmune choreas, metabolic and toxic choreas, and drug-induced choreas. The aim of this chapter is to provide an overview of Sydenham’s chorea (SC) and other main causes of nonhereditary choreas, discussing their clinical features, etiology and pathogenesis, and management. As they represent separate entities, each category will be discussed separately although always following the same outline: etiology, pathophysiology, clinical features, diagnosis, and management.
EPIDEMIOLOGY
Although there are very few community-based studies available regarding the prevalence and incidence of choreas as a whole, there is information regarding the situation in tertiary care centers. According to a study from Pennsylvania, SC accounts for almost 100% of acute cases of chorea seen in children (2). Studies from Australia confirm that it is a relatively common cause of acute chorea in children (3,4). In contrast, the situation is quite distinct in adult patients. Although no published data are available, it is likely that levodopa-induced chorea in Parkinson’s disease (PD) patients is the most common cause of chorea seen by neurologists. One study of consecutive patients seen at a tertiary hospital found that stroke accounted for 50% of all cases, drug abuse was identified in one-third of the patients, and the remaining patients had chorea related to AIDS and other infections as well as metabolic problems (5).
SYDENHAM’S CHOREA
SC, the most common form of autoimmune chorea worldwide, is a major feature of acute rheumatic fever (ARF), a nonsuppurative complication of group A β-hemolytic Streptococcus infection. Despite the decline of ARF, it remains as the most common cause of acute chorea in children in the United States and a major public health problem in developing areas of the world. Clinically, it is characterized by a combination of chorea, other movement disorders, behavioral abnormalities, and cognitive changes (1,2,6).
ETIOLOGY AND PATHOGENESIS
Taranta and Stollerman established the casual relationship between infection with group A β-hemolytic streptococci and the occurrence of SC (7). Based on the assumption of molecular mimicry between streptococcal and central nervous system antigens, it has been proposed that the bacterial infection in genetically predisposed subjects leads to formation of cross-reactive antibodies that disrupt the basal ganglia function. Several studies have demonstrated the presence of such circulating antibodies in 50% to 90% of patients with SC (8,9). A specific epitope of streptococcal M proteins that cross-react with basal ganglia has been identified (10). In one study, it was demonstrated that all patients with active SC have antibasal ganglia antibodies demonstrated by ELISA and Western Blot. In subjects with persistent SC (duration of disease greater than 2 years despite best medical treatment), the positivity was about 60% (11). Recently, it was determined that neuronal tubulin is the target of antineuronal antibodies (12). It must be emphasized that the biologic value of the antibasal ganglia antibodies remains to be determined. One study suggests that they may interfere with neuronal function, however. Kirvan and colleagues (13) demonstrated that IgM of one patient with SC induced expression of calcium-dependent calmodulin in a culture of neuroblastoma cells. Our finding that there is a linear correlation between the increase of intracellular calcium levels in PC12 cells and antibasal ganglia antibody titer in the serum from SC patients further strengthens the hypothesis that these antibodies have a pathogenic value (14). More recent data lend additional support to the molecular mimicry hypothesis. First, we have demonstrated that infusion of sera of SC patients in rodents with 6-OH-dopamine–induced unilateral lesion of the nigrostriatal system caused circling behavior similar to apomorphine (15). This finding suggests that the circulating antibodies act on dopamine (DA) receptors. In another study, rats exposed to Streptococcus antigens not only developed behavioral abnormalities reminiscent of SC but also had IgG that reacted with tubulin, and D1 and D2 receptors, causing elevated calcium/calmodulin-dependent protein kinase II signaling (16). This same group just demonstrated that the injection of these antibodies in the striatum of naïve rats led to behavioral and immunologic abnormalities similar to those found in animals exposed to Streptococcus antigens. Specifically, the antibodies bind to D1 and D2 as well as 5HT-2A and 5HT-2C receptors (17). Interestingly, monoclonal antibodies derived from SC patients target dopaminergic neurons in transgenic mice and signal D2 receptors (18); and there is a correlation between rates on the Universidade Federal de Minas Gerais (UFMG) Sydenham’s Chorea Rating Scale (USCRS) with the ratio between anti-D1 and anti-D2 receptors antibodies (19).
| Nongenetic Causes of Chorea |
Immunologic
• Sydenham’s chorea and variants (chorea gravidarum and contraceptive-induced chorea)
• Systemic lupus erythematosus
• Antiphospholipid antibody syndrome
• Paraneoplastic syndromes
• Acute disseminated encephalomyelopathy
• Celiac disease
Drug-Related
• Amantadine
• Amphetamine
• Anticonvulsants (carbamazepine, hidantoin, lamotrigine, valproic acid)
• Carbon monoxide
• Calcium channel blockers (cinnarizine, flunarizine)
• CNS stimulants (methylphenidate, pemoline, cyproheptadine)
• Cocaine
• Dopamine agonists
• Dopamine-receptor blockers
• Ethanol
• Levodopa
• Levofloxacin
• Lithium
• Sympathomimetics
• Theophylline
• Tricyclic antidepressants
Infections
• AIDS related (toxoplasmosis, progressive multifocal leukoencephalopathy, HIV encephalitis)
• Bacteria
• Diphtheria
• Scarlet fever
• Whooping cough
• Encephalitis
• B19 parvovirus
• Japanese encephalitis
• Measles
• Mumps
• West Nile River encephalitis
• Others
• Parasites
• Neurocysticercosis
• Protozoan
• Malaria
• Syphilis
Endocrine-Metabolic Dysfunction
• Adrenal insufficiency
• Hyper/hypocalcemia
• Hyper/hypoglycemia
• Hypomagnesemia
• Hypernatremia
• Liver failure
Vascular
• Postpump chorea (cardiac surgery)
• Stroke
• Subdural hematoma
Miscellaneous
• Anoxic encephalopathy
• Cerebral palsy
• Kernicterus
• Multiple sclerosis
• Normal maturation (less than 12 months old)
• Nutritional (e.g., B12 deficiency)
• Post-traumatic (brain injury)
Although some investigations suggest that susceptibility to rheumatic chorea is linked to human leukocyte antigen-linked antigen expression (20), there are studies failing to identify any relationship between SC and human leukocyte antigen class I and II alleles (21). An investigation has shown, however, that there is an association between HLA-DRB1*07 and recurrent streptococcal pharyngitis and rheumatic heart disease (22). The genetic marker for ARF and related conditions would be the B-cell alloantigen D8/17 (23). Despite repeated reports of the group that developed the essay claiming its high specificity and sensitivity (24,25), findings of other authors suggest that the D8/17 marker lacks specificity and sensitivity (1). Another suggested genetic risk factor for development of ARF but not SC is polymorphisms within the promoter region of the tumor necrosis factor α gene (26).
Because of the difficulties with the molecular mimicry hypothesis to account for the pathogenesis of SC, there have been studies that address the role of immune cellular mechanisms in this condition. Investigating sera and CSF samples of patients of the Movement Disorders of the Federal University of Minas Gerais, Church and colleagues found elevation of cytokines that take part in the Th2 (antibody-mediated) response, interleukins 4 (IL-4) and 10 (IL-10), in the serum of acute SC in comparison to persistent SC (27). They also described IL-4 in 31% of the CSF of acute SC, whereas just IL-4 was raised in the CSF of persistent SC. The authors concluded that SC is characterized by a Th2 response. However, as they have found an elevation of interleukin 12 in acute SC and, more recently, as we described an increased concentration of chemokines CXCL9 and CXCL10 in the serum of patients with acute SC (28), it can be concluded that Th1 (cell-mediated) mechanisms may also be involved in the pathogenesis of this disorder. A recent investigation confirmed that cellular immune mechanisms might be relevant to the pathogenesis of SC because there is a dysfunction of monocytes (29).
Currently, the weight of evidence suggests that the pathogenesis of SC is related to circulating cross-reactive antibodies. It has been demonstrated that Streptococcus-induced antibodies can be associated with a form of acute disseminated encephalomyelitis characterized by a high frequency of dystonia and other movement disorders as well as basal ganglia lesions on neuroimaging (30). Antineural and antinuclear antibodies have also been found in patients with Tourette’s syndrome (TS), but their relationship with prior Streptococcus infection remains equivocal (31). At the present time, however, there is no conclusive evidence that antibasal ganglia antibodies induced by Streptococcus play a significant role in the pathogenesis of tic disorders. In fact, a population-based epidemiologic survey performed in London failed to demonstrate a significant relationship between streptococcal infection and motor or behavioral syndromes (32,33). More recent studies failed to find immunologic abnormalities that distinguish controls from patients with TS and who meet criteria for PANDAS (34,35).
It remains unclear why up to 50% of patients with SC develop a persistent course of the illness. In this subset of individuals, the titers of antibasal ganglia antibodies are low. Taking into account this finding as well as our observation that serum BDNF levels are high in this group of patients, one may hypothesize that the acute immune process causes structural brain lesions, resulting in permanent dysfunction of the basal ganglia (36).
CLINICAL FEATURES
The usual age at onset of SC is 8 to 9 years, but there are reports on patients who developed chorea during the third decade of life. In most series, there is a female preponderance (28). Typically, patients develop this disease 4 to 8 weeks after an episode of group A β-hemolytic streptococcal pharyngitis. It does not occur after streptococcal infection of the skin. The chorea spreads rapidly and becomes generalized, but 20% of patients remain with hemichorea (37,38). Patients display motor impersistence, particularly noticeable during tongue protrusion and ocular fixation. The muscle tone is usually decreased; in severe and rare cases (8% of all patients seen at the Movement Disorders of the Federal University of Minas Gerais, Brazil), this is so pronounced that the patient may become bedridden (chorea paralytica).
Patients often display other neurologic and nonneurologic symptoms and signs. There are reports of common occurrence of tics in SC. It is, however, virtually impossible to distinguish simple tics from fragments of chorea. Even vocal tics, found in 70% or more of patients with SC in one study, are not of simple diagnosis in patients with hyperkinesias (39). In a cohort of 108 SC patients carefully followed up at our unit, we have identified vocalizations in just 8% of subjects. We have avoided the term “tic” because there was no premonitory sign or complex sound, and conversely, the vocalizations were associated with severe cranial chorea. Taken together, these findings suggest that involuntary sounds present in a few patients with SC result from choreic contractions of the upper respiratory tract muscles rather than true tics (40,41). There is evidence that many patients with active chorea have hypometric saccades, and a few of them also show oculogyric crisis. Dysarthria is common, and there is also impairment of verbal fluency. In fact, a case–control study of patients described a pattern of decreased verbal fluency that reflected reduced phonetic, but not semantic, output (42). This result is consistent with dysfunction of the dorsolateral prefrontal–basal ganglia circuit. Recently studying adults with SC, we have extended this finding, showing that many functions dependent on the prefrontal area are impaired in these patients. The conclusion of this study is that SC should be included among the causes of dysexecutive syndrome (43,44). The prosody is also affected in SC. One investigation of 20 patients with SC has shown decreased vocal tessitura and increased duration of the speech (45–47). Interestingly, these findings are similar to those observed in PD (48). In a survey of 100 patients with rheumatic fever, half of whom had chorea, we found that migraine is more frequent in SC (21.8%) than in normal controls (8.1%, p = 0.02) (49). This is similar to what has been described in TS (50). In the older literature, there are also references to papilledema, central retinal artery occlusion, and seizures in a few patients with SC.
Attention has also been drawn to behavioral abnormalities in SC. Swedo and colleagues found obsessive–compulsive behavior in 5 of 13 SC patients, 3 of whom met criteria for obsessive–compulsive disorder, whereas no patient of the rheumatic fever group presented with obsessive–compulsive behavior (51). In another study of 30 patients with SC, Asbahr and colleagues (52) demonstrated that 70% of the subjects presented with obsessions and compulsions, whereas 16.7% of them met criteria for obsessive–compulsive disorder. None of the 20 patients with ARF without chorea had obsessions or compulsions. These results, however, were roughly replicated by a more recent study that found that patients with ARF without chorea had more obsessions and compulsions than healthy controls (53). This study also tackled the issue of hyperactivity and attention-deficit disorder in SC and found that 45% of their 22 patients met criteria for this condition. Maia and colleagues investigated behavioral abnormalities in 50 healthy subjects, 50 patients with rheumatic fever without chorea, and 56 patients with SC (54). The authors found that obsessive–compulsive behavior, obsessive–compulsive disorder, and attention-deficit and hyperactivity disorder were more frequent in the SC group (19%, 23.2%, 30.4%) than in the healthy controls (11%, 4%, 8%) or in the patients with ARF without chorea (14%, 6%, 8%). In this study, the authors demonstrated that obsessive–compulsive behavior displays little degree of interference in the performance of the activities of daily living. Another study compared the phenomenology of obsessions and compulsions of patients with SC with subjects diagnosed with tic disorders. The authors demonstrated that the symptoms observed among the SC patients were different from those reported by patients with tic disorders but were similar to those previously noted among samples of pediatric patients with primary obsessive–compulsive disorder (55). Another investigation comparing healthy controls with patients with rheumatic fever showed that obsessive–compulsive behavior is more commonly seen in patients with SC with relatives who also have obsessions and compulsions (56). This study makes clear that there is interplay between genetic factors and environment in the development of behavioral problems in SC. In a careful investigation of psychiatric comorbidities in 50 patients with SC, we found that the most frequent psychiatric disorders observed in SC patients were major depression (14%); generalized anxiety disorder (16%), social phobia (24%), and obsessive–compulsive disorder (24%). The frequency of psychiatric disorders did not differ between SC patients in remission in comparison with patients with persistent chorea, except for depressive disorders which were more frequent in the latter (57). We also reported that although rarely, SC may induce psychosis or trichotillomania during the acute phase of the illness (58,59).
An investigation demonstrated that the peripheral nervous system is not targeted in SC (60). Finally, it must be kept in mind that SC is a major manifestation of rheumatic fever. Sixty percent to 80% of patients display cardiac involvement, particularly mitral valve dysfunction, in SC, whereas the association with arthritis is less common, seen in 30% of patients; however, in approximately 20% of patients, chorea is the sole finding (38,61). A prospective follow-up of patients with SC with and without cardiac involvement in the first episode of chorea suggests that the heart remains spared in those without lesion at the onset of the rheumatic fever (62).
DIAGNOSIS
The current diagnostic criteria for SC are a modification of the Jones criteria: chorea with acute or subacute onset and lack of clinical and laboratory evidence of an alternative cause. The diagnosis is further supported by the presence of additional major or minor manifestations of rheumatic fever (42,63,64). Of note, according to the current criteria, the diagnosis of SC is still possible in the absence of any other feature of RF.
The USCRS, the first validated scale to rate SC has been published, provides a detailed quantitative description of the performance of activities of daily living, behavioral abnormalities, and motor function of patients with SC. It comprises 27 items, and each one is scored from 0 (no symptom or sign) to 4 (severe disability or finding) (65). It is important to emphasize that the USCRS is not intended to be used as a diagnostic tool but rather to assess patients already with an established diagnosis of SC.
Several conditions may present with clinical manifestations similar to SC (1). The most important differential diagnosis is systemic lupus erythematosus (SLE), which will be discussed later in this chapter. From a clinical point of view, the majority of subjects with SLE will have other nonneurologic manifestations such as arthritis, pericarditis, and other serositis as well as skin abnormalities. Moreover, the neurologic picture of SLE tends to be more complex and may include psychosis, seizures, other movement disorders, and even mental status and consciousness level changes. Only in rare instances will chorea, with a tendency for spontaneous remissions and recurrences, be an isolated manifestation of SLE. The difficulty in distinguishing these two conditions is increased since by the finding that at least 20% of patients with SC display recurrence of the movement disorder. Eventually, patients with SLE will develop other features, meeting diagnostic criteria for this condition (1). Primary antiphospholipid antibody syndrome (PAPS) is differentiated from SC by the absence of other clinical and laboratory features of RF as well as the usual association with repeated abortions, venous thrombosis, other vascular events, and the presence of typical laboratory abnormalities. Anti-N-methyl-D-aspartate receptor encephalitis is a condition that has become increasingly diagnosed. In children and adolescents, it is usually a paraneoplastic disorder related to ovarian teratoma. Patients present with a combination of psychiatric problems and a mixed movement disorder that may include chorea. However, the severe and proteiform clinical presentation readily distinguishes this condition from SC (66). Encephalitides, either as a result of direct viral invasion or by means of an immune-mediated postinfectious process, can cause chorea (67). This usually happens, however, in younger children; the clinical picture is more diversified to include seizures, pyramidal signs, and impairment of the psychomotor development. There are also laboratory abnormalities suggestive of the underlying condition. Drug-induced choreas are readily distinguished by careful history demonstrating a temporal relationship between onset of the movement disorder and exposure to the agent. Although vascular disease is uncommon in the first two decades of life, it can occur and cause chorea (68). This may happen in 3% patients with moyamoya disease (69). Benign hereditary chorea is another condition that causes chorea in children and may mimic SC. Despite the fact that it is an autosomal dominant disorder, due to the variable expression the family history may remain unnoticed. These patients display chorea, other movement disorders (tics, myoclonus) as well as behavioral disorders (70).
Children and young adults with chorea should undergo complete neurologic examination and diagnostic testing to determine the etiology of various causes of chorea. As there is no specific biologic marker of SC, the aim of the diagnostic workup in patients suspected to have rheumatic chorea is threefold: (1) to identify evidence of recent streptococcal infection or acute phase reaction, (2) to search for cardiac injury associated with RF, and (3) to rule out alternative causes. Tests of acute phase reactants such as erythrocyte sedimentation rate, C-reactive protein, leukocytosis, and other blood tests like rheumatoid factor, mucoproteins, protein electrophoresis, and supporting evidence of preceding streptococcal infection (increased antistreptolysin-O, antiDNAse-B, or other antistreptococcal antibodies; positive throat culture for group A Streptococcus; recent scarlet fever) are much less helpful in diagnosing SC than in other forms of RF due to the usual long latency between the infection and onset of the movement disorder. Elevated antistreptolysin-O titer may be found commonly in populations with a high prevalence of streptococcal infection. Furthermore, the antistreptolysin-O titer declines if the interval between infection and rheumatic fever is greater than 2 months. Anti-DNase-B titers, however, may remain elevated up to 1 year after strep pharyngitis. Heart evaluation (i.e., doppler echocardiography) is mandatory because the association of SC with carditis is found in up to 80% of patients. Cardiac lesions are the main source of serious morbidity in SC. Serologic studies for SLE and PAPS must be ordered to rule out these conditions. EEG has little importance in the evaluation workup of these patients, showing nonspecific generalized slowing acutely or after clinical recovery. Spinal fluid analysis is usually normal, but it may show a slight increased lymphocyte count. In general, neuroimaging will help rule out vascular and other structural causes such as moyamoya disease. CT scan of the brain invariably fails to display abnormalities. Similarly, head MRI is often normal, although there are case reports of reversible hyperintensity in the basal ganglia area. In one study, the authors showed increased signal in just 2 of 24 patients, although morphometric techniques revealed mean values for the size of the striatum and pallidum larger than controls (71). Unfortunately, these findings are of little help on an individual basis because there was an extensive overlap between controls and patients. PET and SPECT imaging may prove to be useful tools in the evaluation, revealing transient increases in striatal metabolism during the acute phase of the illness, a finding confirmed by a recent study (71–76). Interestingly, in a recent study we demonstrated that hypermetabolism on SPECT remains even in patients with remission of motor features of SC (77) (Fig. 20.1). This contrasts with other choreic disorders (such as Huntington’s disease) that are associated with hypometabolism. Of note, however, a recent investigation showed hyperperfusion in two patients with SC whereas the remaining five had hypometabolism (78). It is possible that the inconsistencies in these studies reflect heterogeneity of the population of patients. An increasing interest is now directed to autoimmune markers that may eventually be useful for diagnosis. The test of antineuronal antibodies, however, is not commercially available, being just performed for research purposes. Preliminary evidence, moreover, suggests that these antibodies are not specific for SC. Similarly, the low sensitivity and specificity of the alloantigen D8/17 renders it unsuitable as a diagnostic test.
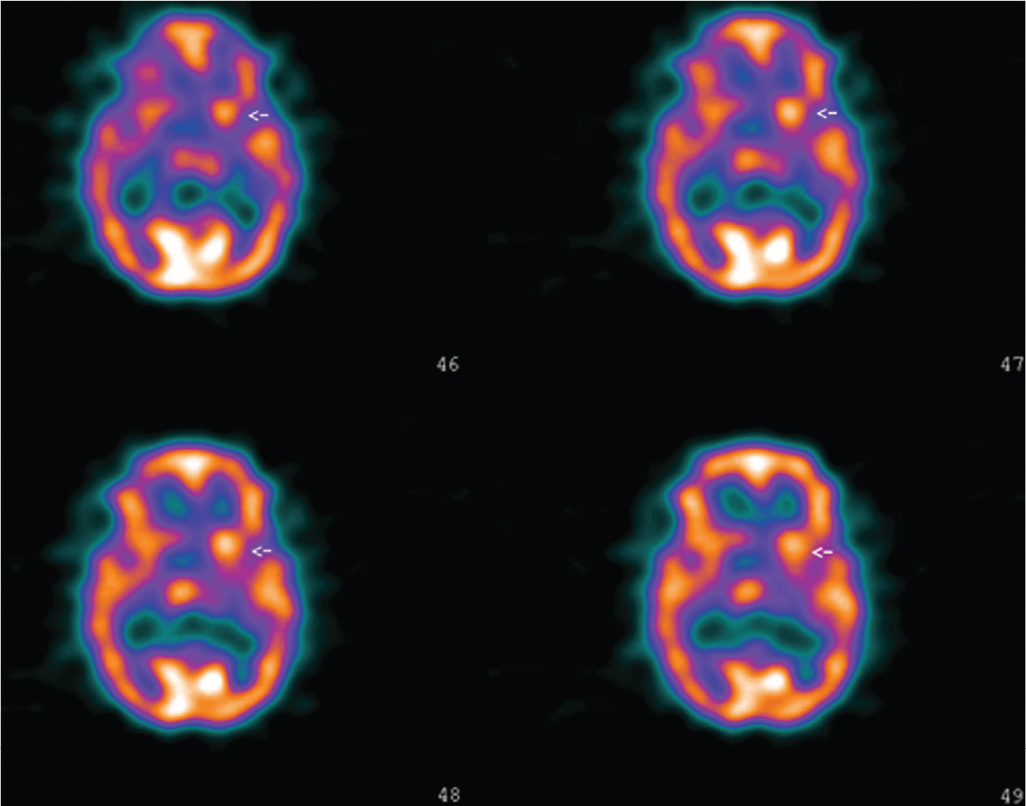
Figure 20.1. Perfusion SPECT of a patient with acute right hemichorea caused by Sydenham’s chorea. There is hyperperfusion of the left striatum.








