CHAPTER 239 Techniques and Options in Nerve Reconstruction and Repair
Anatomic Principles
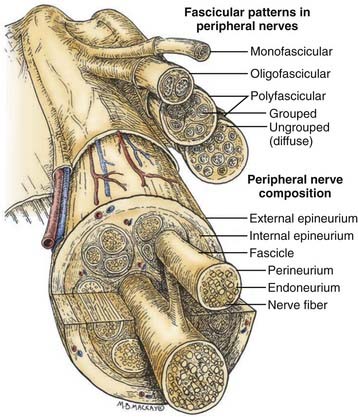
Before one can even consider the repair of a peripheral nerve, an understanding of the connective tissue layers, as well as of the fascicular anatomy of a nerve, is important. As seen in Figure 239-1, which contains a diagram of the peripheral nerve architecture and its components, an external epineurial sheath, composed of connective tissue and longitudinal blood vessels, surrounds each peripheral nerve. There is an external epineurium and an internal epineurium. The internal epineurium demarcates fascicles and groups of fascicles within the nerve. Each individual fascicle is surrounded by perineurium. The axons themselves are contained within fascicles, in close association with Schwann cells and the basement membrane that surrounds Schwann cells, the endoneurial basal lamina (also referred to as endoneurial tubes).1
Nerves may be generally divided into four basic patterns of intraneural architecture, based on their fascicular structure (see Fig. 239-1).2 Nerves containing one large fascicle are termed monofascicular, whereas those containing a few or discrete number of fascicles are oligofascicular. Most nerves contain many fascicles of varying sizes and are termed polyfascicular. The polyfascicular pattern may exist with grouping of fascicles or with a more diffuse (ungrouped) arrangement throughout the cross section of the nerve. The fascicular nature of a nerve changes as it extends from proximal to distal in the extremity. For example, the ulnar nerve is polyfascicular as it comes off the brachial plexus and then generally becomes organized into usually four fascicles at the level of the elbow. These fascicles are further segregated into motor and sensory groupings at the level of the wrist, and finally, the terminal digital branches are monofascicular in the fingers.
The proportion of connective tissue within the nerve varies considerably, from 25% to 85%, across the cross section of the nerve.2 In general, there is more connective tissue in the nerve where it crosses the joint. The connective tissue, particularly the perineurium, is the source of the main tensile strength to the nerve. It is also the layer that can take and hold a suture. From a practical viewpoint, the smallest component of nerve that can therefore be repaired using current microsurgical technique is the fascicle.
It must be stressed at the outset that a peripheral nerve repair is not a type of cellular repair but is actually a repair done at the level of the connective tissue to coapt a healthy proximal nerve to a healthy distal nerve stump. This then provides the appropriate anatomic environment so that axons from the proximal stump can regenerate into endoneurial tubes within the distal nerve stump and, hence, be led to end organs to restore function. Note that a nerve graft functions as a conduit, whose axons are destined to undergo wallerian degeneration as soon as it is removed from its harvest site. Thus, the graft provides an endoneurial tube network available to be exploited by regenerating axons from the proximal host nerve stump.3 It also provides viable Schwann cells, as long as the caliber of the nerve graft is not too large. For this reason, small caliber cutaneous nerves are most commonly used as graft material (see later section on donor graft harvesting techniques). The small caliber nerves, when sutured in a series of parallel segments, are in close proximity to tissue fluid and are therefore nourished. They also undergo rapid revascularization and thus remain viable.
Physiologic Principles and Patient Selection for Surgery
Peripheral nerves, once injured, have the potential to regenerate axons and reinnervate end organs, with resulting good functional recovery.4 Indeed, this is the case with all minor nerve injuries, such as neurapraxia, in which the axon remains intact. After a nerve injury resulting in axotomy (Sunderland grade II injury or greater), the distal axon undergoes wallerian degeneration. In purely axonotmetic injuries, in which axons are interrupted but the degree of connective tissue damage is minimal, regenerating axons use their existing endoneurial pathways to specifically reinnervate their own precise target end organs, as confirmed in recent experiments using bioengineered fluorescent mice.5 Outcome after more severe peripheral nerve injury, however, remains variable and often very poor. Most of these injuries exhibit both a loss of axon continuity and a significant disruption in the internal connective tissue structures. The resulting scarring within the nerve or a frank gap (with lacerating injuries) presents a formidable barrier to regenerating axons, preventing them from effectively innervating the distal nerve stump. These are currently managed with a repair of the divided nerve or, for the usual scenario of longer gaps or scar segments that need to be resected, placement of interposed nerve grafts.
Simplistically, exploration and repair of the peripheral nerve is indicated in clinical situations in which there is either the absence or the lack of expectation that there will be effective spontaneous regeneration. This will be the case in all patients with lacerating nerve injuries and in many of the patients who harbor the more severe injuries in continuity. As a practical rule, nerves known or expected to be sharply lacerated should be explored and repaired primarily and without delay, whereas bluntly lacerated nerves should be repaired after a period of 2 to 4 weeks. Patients with nerve injuries in continuity should be followed for about 3 months with repeat clinical and electrophysiologic evaluation. After 1 to 2 months have elapsed from the time of the trauma, the initial effects of any tissue damage will have resolved, and magnetic resonance neurography may then provide an early view of neuroma formation or of complete discontinuity. In patients failing to demonstrate clinical or electrical evidence of regeneration, the nerve should be explored within 4 to 6 months.6 The findings at surgery, including intraoperative electrophysiologic tests (briefly discussed later but in detail elsewhere in this text), determine the fate of the nerve injury (neuroma)–in-continuity.7
Operative Principles and Fundamental Techniques
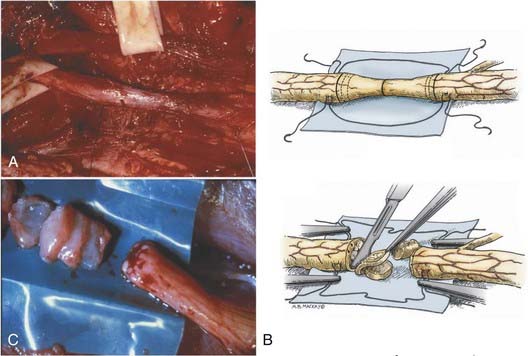
Isolation of the nerve itself should be performed using sharp dissection. The surgeon identifies normal nerve proximal and distal to the zone of injury and then works toward the area of injury. In clean lacerating injuries, the area of exposure may be relatively small. However, most injuries leave the nerve in continuity; because these injuries are also explored weeks to months following trauma, there is considerable scar formation and distortion of tissue, necessitating a wide and extensile exposure. Using sharp dissection techniques, the area of injured nerve is circumferentially exposed; that is, an external neurolysis is performed. With this type of circumferential mobilization, the gross anatomic details of the injury are identified. With the aid of an operating microscope, finer anatomic details can be appreciated. If the nerve is in clear discontinuity, nerve repair is necessary. However, most lesions are in-continuity. As demonstrated by Kline and Happel, recording of intraoperative nerve action potentials is useful in assessing these lesions.8 Specifically, the presence of a nerve action potential across the lesion argues for the lesion not to be resected (Fig. 239-2A). However, the lack of evidence of spontaneous regeneration (the absence of a nerve action potential) dictates resection of the neuroma and appropriate reconstruction of the resulting nerve injury gap.
The placement of lateral stay sutures using 6-0 monofilament (as illustrated in Fig. 239-2A and B) helps maintain the topographic alignment of the nerve. Under the operating microscope, the surgeon then cuts across the center of the neuroma. Small segments of the nerve are sliced in perfect cross section, using a fresh blade, until a healthy fascicular pattern is identified both at the proximal and at the distal stump9 (Fig. 239-2B and C). This step is critical because attempting to appose or graft scarred proximal and distal stumps is a major cause of nerve repair failure. Healthy fascicular tissue is recognized when the epineurium retracts slightly and the endoneurium appears to “pout” or mushroom out of the fascicles (because of positive endoneurial pressure). Fine bleeding from endoneurial microvessels may also be appreciated. This type of adequate débridement invariably leaves some degree of gap between the proximal and the distal stump. If the gap is short and the two ends can be brought together without undue tension, a direct repair is appropriate. One good way to determine the degree of tension present at the suture line is to bring the ends together using the stay epineurial sutures. If this can be performed without suture distraction, a direct repair is appropriate. However, if the ends are under considerable tension and the suture line appears to tear out, a graft repair must be performed.
Nerve Repair Techniques
Methods of peripheral nerve repair fall under two basic categories: direct repair (neurorrhaphy) and bridge procedures, in which most commonly, autologous nerve grafts are used. The suture repair may be performed using an epineurial, group fascicular, or fascicular technique or various combinations of these methods.10
Direct Repair
Epineurial Repair
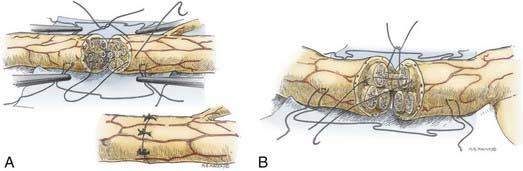
Epineurial suture repair has been a traditional method of nerve coaptation. These repairs are most appropriate for monofascicular (e.g., digital) nerves and diffusely grouped polyfascicular (most proximal limb and plexus element) nerves. Simplistically, this method achieves continuity of the connective tissue from the proximal to the distal stumps, without tension and with appropriate rotational alignment of both stumps. The goal is to obtain good coaptation of proximal and distal fascicular anatomy. Freshening of the two nerve ends to débride the nerve and remove scar tissue is therefore critical. Achieving appropriate nerve alignment can be aided by inspecting for longitudinal blood vessels in the epineurium as well as attending to fascicular alignment. The use of lateral stay sutures (see Fig. 239-2) also aids this process. Neurorrhaphy is performed using 8-0 to 10-0 nonabsorbable nylon sutures. A small bite of the internal and the external epineurium (being careful to avoid perineurium) is taken from both stumps, and the suture is tied using only mild to moderate tension (Fig. 239-3A). It is critical to avoid tying the knot under too great a tension because this will cause overriding or an accordion effect on the fascicles or, in fact, pouting out of a fascicle from the epineurial repair site, thus defeating the purpose of suturing. Two initial sutures are placed 180 degrees apart. If needed, this distance is then divided in half, and two further sutures are positioned. The number of epineurial sutures required varies; in most cases, four to eight sutures suffice for approximating the proximal and the distal stumps in a tension-free manner. Excess sutures may result in additional scarring and are to be avoided.
Grouped Fascicular Repair
As in the epineurial repair method, the nerve ends are matched by resecting damaged tissue. Débridement is followed by careful analysis, under the operating microscope, of the anatomic cross-sectional appearance of the nerve stump. Using the cross-sectional appearance, the longitudinal blood supply and other spatial landmarks (e.g., branching of nerve just proximal and distal to the injury site), the proximal and distal stumps can be matched. Interfascicular dissection is then performed within the internal epineurium to draw out groups of fascicles (Fig. 239-3B). Groups of fascicles may vary from two to several, each surrounded by a variable amount of internal epineurium, with the external epineurium dissected away. After groups of fascicles are adequately matched, 8-0 or 9-0 microsutures are placed through the interfascicular epineurial tissue and perineurium, allowing coaptation of fascicular groups from the proximal to the distal stump (see Fig. 239-3B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree