The EEG in Inflammatory CNS Conditions
Robert L. Beach
Helen Barkan
Edgar Deperalta
ACKNOWLEDGMENTS
This chapter addresses the electroencephalogram in infectionrelated and immune-mediated CNS inflammatory conditions. This distinction is somewhat artificial in itself, since most CNS inflammation is immune-mediated, either primarily or secondarily. Previous versions of this chapter were written by the second author’s most esteemed mentor, Dr. Barbara Westmoreland. The current version is an update, which reflects significantly her work and wisdom. Since the last edition, the information on the etiology of several immune-mediated CNS inflammatory processes has progressed significantly, and their associated electroencephalographic findings are reviewed here. Some previous topics are covered in other chapters of this volume.
INTRODUCTION
The range of EEG abnormalities in inflammatory and infectious disorders was the subject of great interest in the 1950s and 1960s, but has not received as much attention since then. The main reason for this lack of engagement is the improvement in diagnostic imaging modalities, and the resultant recognition of the limitations of the use of EEG in the diagnosis, treatment, and follow-up of most of those conditions, with a few notable exceptions. The EEG of an inflamed brain can be completely normal, can reveal minor generalized or lateralized abnormalities, or may show severe focal or global slowing, and may also contain a variety of potentially epileptogenic abnormalities or frank electrographic seizures of focal, lateralized, or generalized onset. Hence, many believe that most of those EEG abnormalities are nonspecific, and of low to moderate diagnostic and prognostic value, except for a few “signature” EEG patterns corresponding to specific conditions and even to specific pathogens. More EEG-based research directed specifically at validating the prognostic and diagnostic value of the EEG in infectious and inflammatory CNS conditions is clearly needed. In the digital EEG age, and with sophisticated analysis software, sensitive feature recognition should allow the revitalization of this pursuit.
The majority of EEG changes in inflammatory and infectious CNS disease processes represent the “final common pathway” of the electrographic activity of the inflamed cortex in either primary or secondary inflammation. Nevertheless, it is important to recognize both the specific and the nonspecific patterns, and to use them as aid to diagnosis, to gauge the response to treatment, and for prognostic purposes as well.
For consistency, all EEG tracings in the figures illustrating the text below were captured at the amplitude of 5µ V/mm, with 1- to 70-Hz bandpass filter, and with the notch filter on, with about 13 seconds per page.
PART 1: INFECTIOUS CONDITIONS
Meningitis
Infection and inflammation of the meninges can have bacterial, fungal, parasitic, autoimmune, and malignancy-related pathogenesis, or it can be a secondary to a brain abscess or empyema with contiguous spread of infection. In the latter case, lateralizing EEG findings can be anticipated. In the rest of the conditions, the EEG may range from completely normal to severely abnormal including focal- or generalized-onset seizures and potentially epileptogenic discharges. However, in the majority of these conditions, only diffuse slow-wave abnormalities of variable severity will be seen. There is objective evidence (1), as cited in the previous version of this chapter (2), that:
Although the electroencephalographic findings are not essential for making the specific diagnosis of meningitis, the EEG and particularly serial recordings are helpful in following the course of the disease, detecting the development of complications or relapse, and indicating the presence of sequelae of residual brain damage.
Purulent Meningitis
Meningococcal, streptococcal, and staphylococcal meningitis are usually community-acquired, with pneumococcal meningitis being an infrequent complication of pneumonia, otitis media, mastoiditis, severe alcoholism, and CSF leakage after ENT procedures. Staphylococcal meningitis may complicate postoperative course of cranial surgery.
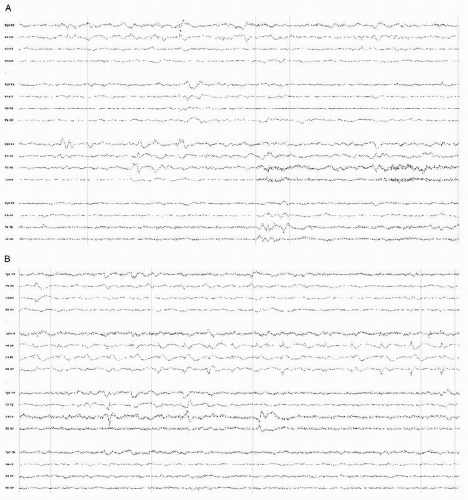
Diffuse moderate to very severe and nonspecific slow-wave abnormalities are typical for the EEG in those conditions (Fig. 18.1) (3). The slowing is frequently prominent bifrontally, resembling FIRDA (frontal intermittent rhythmic delta activity) possibly associated with increased intracranial pressure (Fig. 18.2).
Studies of initial EEG evaluation of patients with purulent bacterial meningitis suggest that about one half of the patients have slow-wave abnormalities or electrographic seizures (3).
If only community-acquired disease is considered, about 20% of the patients will have clinical seizures, and a small number of those have clinical and electrographic status epilepticus (4). The patients with seizures have mortality of almost three times higher than those without the seizures. Focal radiographic lesions are found in one third of patients with meningitis and in-hospital seizures.
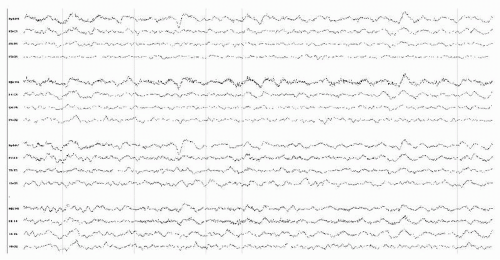 Figure 18.1 Awake EEG of a 70-year-old man with community-acquired staphylococcal meningitis showing diffuse moderately severe delta slowing. |
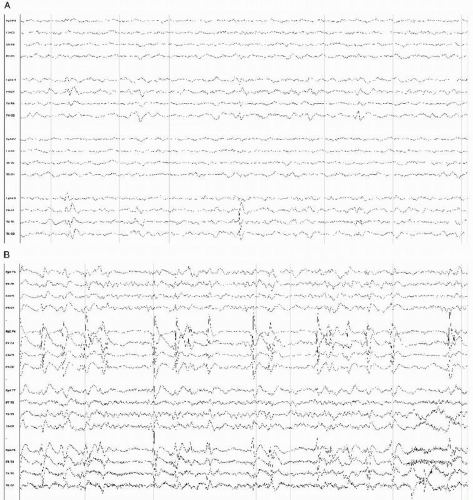
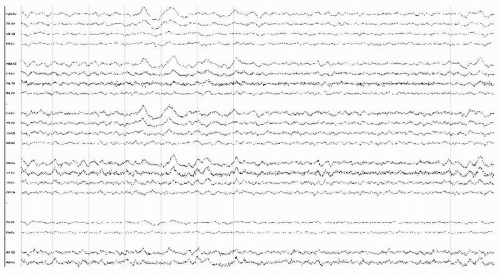 Figure 18.2 EEG in an obtunded 63-year-old man with cervical MRSA abscess and secondary meningitis, showing rhythmic bifrontal slowing consistent with FIRDA. |
Other EEG abnormalities in bacterial meningitis also have been described, such as triphasic waves and FIRDA, although both are more common for meningitis of other etiologies.
An attempt to predict the bacterial or viral nature of the pathogen from the EEG abnormalities suggests that moderately severe slowing in the delta range is much more likely in bacterial meningitis (5).
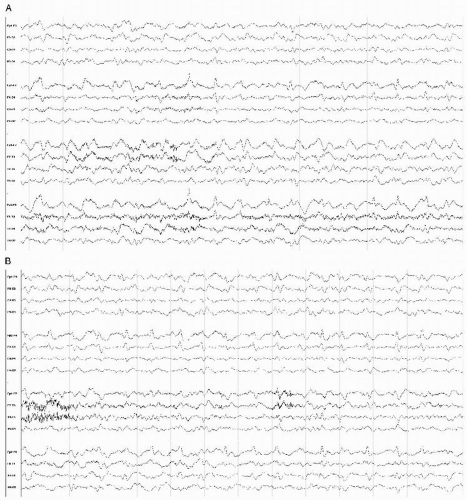
In neonates with meningitis, with group B streptococcus and E. coli being the most common pathogens, the presence of EEG abnormalities and/or electrographic and clinical seizures was directly correlated with prognosis, and associated with poor outcome (Fig. 18.3). Neonatal convulsive disorders of multiple etiologies are covered in a separate chapter of this volume.
Brain abscess and subdural empyema can be either cause or sequelae of bacterial meningitis. The pathogens are often mixed mouth flora, including anaerobes, staphylococci, and gramnegative commensal organisms. The EEG findings in such cases often reflect a focal lesion—with moderately severe slowing, potentially epileptogenic abnormalities, and clinical and/or electrographic seizures. The focal findings may manifest as polymorphic delta slowing, suppression of activity over the area of suppuration, or, infrequently, lateralized polyphasic potentially epileptogenic abnormalities such as sharp waves or periodic lateralized epileptiform discharges (PLEDs).
Basal Meningitis
Basal meningitis due to mycobacteria, Listeria, or spirochetes (syphilis and borreliosis) overlaps with the entity called “granulomatous meningitis,” or “chronic meningitis,” and also with meningoencephalidites described for the same pathogens. The separate use of those terms is imprecise, since biopsy is obtained only in rare cases, and the degree of chronicity varies widely. EEG in basal meningitis ranges from completely normal to severely abnormal, and in addition to varied degrees of generalized slowing, hemispheric asymmetries, and sharp paroxysmal activity that is either focal or generalized, triphasic waves are not uncommon (6), as well as FIRDA similar to that in Figure 18.2 (7), which may reflect the underlying increase in intracranial pressure. Because of the association of those pathogens with cerebral vessel vasculitis, it is rare to see normal EEG in those patients. In one series of 19 patients, 11 had EEG abnormalities. Tuberculous meningitis in another series was associated with similar EEG abnormalities in 24 out of 32 patients.
Focal epileptic discharges and electrographic seizures are also common in tuberculous meningitis (8), particularly in children, and reflect the patchy focal manner of meningeal inflammation.
Fungal and Parasitic Meningitis
These pathogens are relatively uncommon in a healthy host. Immunosuppressed patients with cryptococcal, cystericercal, candidial, or other meningitis—with the inflammation that can be isolated to the meninges, but usually also involving the brain parenchyma—are typically very ill, and their EEG pattern is that of a severe encephalopathy, with polymorphic slowing. Focal seizures and status epilepticus are common in those patients as well as in those with basal meningitis.
Meningitis due to cystocercosis, aspergillosis, nocardiosis, echinococcosis, mycormycosis, toxoplasmosis, and candidiasis is associated with focal slow-wave abnormalities (2), again reflecting the patchy nature of meningeal pathology due to infection by those organisms. No organism-specific patterns are evident, however.
Viral Meningitis
Viral, otherwise known as “aseptic,” meningitis may not result in any EEG abnormalities, or may result in mild to moderate generalized slowing. In one series of 62 patients, 52% had abnormal EEGs with slow-wave abnormalities (9). Other investigators found that only 28% out of 82 patients (10), or in yet other studies, one third of patients with CSF-confirmed aseptic meningitis had EEG abnormalities. Thus, a normal EEG with inflammatory CSF findings strongly suggests viral nature of the pathogen—or early stage of CNS involvement.
Practically any infectious agent can cause mild to moderate transient meningoencephalitis with variable EEG changes. Parenchymal involvement is assumed, since biopsy is rarely available, and thus will be discussed in the section “Encephalitis with Nonspecific EEG Patterns.” Rarely a purely encephalitic process, such as HSV encephalitis, can cause focal meningeal irritation and a clinical and laboratory picture consistent with aseptic or chemical meningitis.
Mollaret Meningitis
The agent of this recurrent, lymphocytic meningitis with relatively benign CSF findings and clinical course was thought to be herpes simplex type II virus, which has been recovered from the CSF in some cases. However, some suggest the term should be restricted to idiopathic cases, as several other infectious and autoimmune etiologies as well as CSF leaks and shunts have been implicated in cases of recurrent meningitis (11, 12, 13 and 14). EEG in Mollaret meningitis is usually normal; however, diffuse moderately severe slowing can be seen, correlated with the clinical symptoms of headache and confusion. Seizures are unusual.
Carcinomatous Meningitis
Involvement of the meninges in the neoplastic process is not an uncommon terminal manifestation of systemic cancer. The EEG in these patients is practically never normal, with moderate or very severe slowing that correlates with the clinical severity of the encephalopathic state. Focal abnormalities such as PLEDs, sharp waves, and spikes are also common. Generalized triphasic periodic waves have also been described.
Meningoencephalitis
Meningoencephalitis is a general term, describing the inflammation of both the meninges and the brain parenchyma due to toxic, metabolic, infectious, or autoimmune etiologies, with many of them overlapping on many occasions. Demyelinating disease, connective tissue diseases, and malignancy-related encephalitides are discussed later in this chapter. This section will concentrate on infectious and autoimmune etiologies exclusive of the above-mentioned.
Similar to the EEG in meningitis, the encephalitic brain can produce a normal or an almost normal study, or display profound abnormalities. The same groups of pathogens that cause meningitis, bacterial, viral, parasitic, and fungal, also commonly cause meningoencephalitis. Dissimilar to viral meningitis, the patients with viral encephalitis of any etiology rarely have a normal EEG. The EEG abnormalities range from diffuse slowing to electrographic seizures, and are of the two varieties—disease-specific and diagnostically vague. There are three of the former variety and hundreds of the latter. Nevertheless, the EEG is of value in both cases, since disease-specific patterns represent common conditions, and the
absence of a specific pattern may in itself be diagnostic. The evolution of encephalitic EEG from the prodromal to acute phase of the disease and to recovery is similar to that in meningitis, and may provide useful information for prognosis, complications, and potential sequelae.
absence of a specific pattern may in itself be diagnostic. The evolution of encephalitic EEG from the prodromal to acute phase of the disease and to recovery is similar to that in meningitis, and may provide useful information for prognosis, complications, and potential sequelae.
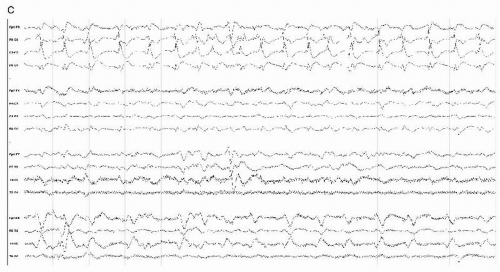 Figure 18.3 (continued) (B and C) with no clinical accompaniment; electrographic seizures with the subtle correlate of contralateral eye deviation were also seen. |
Encephalitis with Disease-Specific EEG Patterns
Only three neurologic infections have a corresponding pathognomic EEG signature: herpes simplex encephalitis, most common variants of prion disease, and subacute sclerosing panencephalitis (SSPE), although the latter is a very rare diagnostic entity in the Western world, where measles vaccination is a norm.
Herpes Simplex (HSV-I) Encephalitis This common catastrophic CNS illness strikes healthy people of all ages, but is more common in patients over 50 years. HSV-1 has a predilection for the temporal lobes and for the frontotemporal areas, as compared to its cousin HSV-2, the presumed causative agent of Mollaret meningitis, which does not usually affect the parenchyma. The classical presentation of intermittent fever, confusion, and seizures with CSF lymphocytosis and positive PCR for the virus is atypical in clinical practice: the diagnosis is best made based on any combination of clinical, radiographic, laboratory, and EEG findings compatible with this condition. Since this is one of the few treatable encephalitides, initial overdiagnosis is preferable in the case of HSV-1 encephalitis.
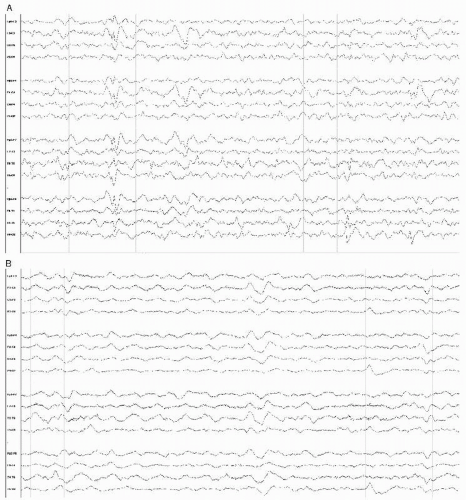
The initial EEG abnormalities (1,15) consist of polymorphic delta slowing, often more prominent over the most involved temporal lobe, with focal sharp complexes appearing and rapidly evolving into pseudoperiodic to periodic PLEDs, 0.3 to 1 Hz in frequency, and di- or triphasic broad or tight giant sharp waves. These may be bilateral (biPEDs), bifrontal, bitemporal, or even generalized (GPEDs). On occasion, PLEDs are focal with a small field in a specific location, such as the anterior temporal region (Fig. 18.4).
Temporally, PLEDs and/or focal sharp complexes reveal themselves within days to a week after the onset of symptoms, but they may persist for several weeks into the illness, and may be correlated with the response to treatment.
In addition, seizure discharges are common over the most affected areas, consisting of rhythmic activity or polyspike bursts. Clinical correlate may or may not occur, but confusion and dysphasia are likely if the dominant hemisphere is affected. Obtundation and coma are not uncommon, especially when EEG abnormalities are severe and widespread. When clinical motor seizures do occur, the frequency of clonic limb activity may not match the frequency of PLEDs, which may be significantly slower than the clonus.
In refractory or fatal cases, a burst suppression pattern may be seen, with generalized EEG attenuation following a burst of PLEDs or polyspikes, which indicates a grim prognosis. In nonfatal HSV encephalitis, the strikingly abnormal EEG recovers slowly to almost normal on serial examinations. The PLEDs become infrequent, intermittent, and are replaced by lateralized slow-wave abnormalities. Clinical improvement often precedes electrographic normalization.
Possibly because of the associated severe encephalopathy, other, seemingly atypical patterns are frequently seen in HSV
encephalitis, including generalized triphasic waves or atypical triphasics, with posterior-to-anterior phase shift, multifocal sharp waves, and severe polymorphic delta slowing.
encephalitis, including generalized triphasic waves or atypical triphasics, with posterior-to-anterior phase shift, multifocal sharp waves, and severe polymorphic delta slowing.
Because the most common reason for PLEDs is a vascular insult, dual etiology needs to be considered as part of the differential diagnosis. The coexistence of HSV-1 encephalitis and stroke or a hemorrhage is common, and radiologic correlation is crucial.
Prion Diseases Creutzfeldt-Jakob disease (CJD), variant CJD or “mad cow disease,” the exceedingly rare fatal familial insomnia (a.k.a. Gerstmann-Straussler-Scheinker disease), and kuru are
the four well-described prion disorders, which can be familial, sporadic, or infectious in etiology. The pathogen is an abnormally folding isoform of a native CNS protein, known as prion protein. The most common variant of classic CJD is sporadic rather than genetic, although many inherited polymorphisms predispose to the disease. Presenting with the clinical manifestations of a rapidly progressive dementia in a person over 60 years, it is often misdiagnosed at early stages as depression, psychosis, or another psychiatric illness. Spontaneous myoclonus and stimulus-related myoclonus come in later stages of the disease, as well as severe generalized spasticity, rhizus sardonicus, obtundation, and death. There are no known cases of recovery.
the four well-described prion disorders, which can be familial, sporadic, or infectious in etiology. The pathogen is an abnormally folding isoform of a native CNS protein, known as prion protein. The most common variant of classic CJD is sporadic rather than genetic, although many inherited polymorphisms predispose to the disease. Presenting with the clinical manifestations of a rapidly progressive dementia in a person over 60 years, it is often misdiagnosed at early stages as depression, psychosis, or another psychiatric illness. Spontaneous myoclonus and stimulus-related myoclonus come in later stages of the disease, as well as severe generalized spasticity, rhizus sardonicus, obtundation, and death. There are no known cases of recovery.
The initial EEG abnormalities are nonspecific and mild, usually consisting of generalized slowing and disorganization of the background (16,17). However, subclinical, that is, nonconvulsive status epilepticus (NCSE) or epilepsia partialis continua (EPC) have been repeatedly described as initial presenting findings in CJD (18, 19, 20, 21, 22 and 23). Over weeks to months, focal di- or triphasic sharp discharges appear, with an after-coming slow component. These are often generalized or bifrontal, occasionally more prominent over one region or hemisphere, without a clinical correlate. As the disease progresses, these become more persistent, severe, and eventually relentless (17).
The pathognomic pattern of CJD in the EEG consists of continuous generalized, bisynchronous periodic stereotypic sharp waves, occurring in intervals of 0.5 to 1 second with duration of 200 to 400 msec. Initially those abnormalities are lateralized; however, they become generalized as disease progresses. Atypical patterns may be seen, with bifrontal sharp waves that are pseudoperiodic, or even with multifocal spike-wave discharges. CSF 14-3-3 protein and MRI abnormalities on DWI and later T2 images in cortex and basal ganglia are usually positive in both typical and atypical cases (Fig. 18.5) (24).
Myoclonic jerks maximal in the upper body may occur in close temporal association with generalized sharp wave complexes, or they may have no EEG correlate. Startle myoclonus or provoked myoclonus is seen on occasion. Occasionally, external stimuli can drive the rate of both the myoclonus and the corresponding sharp wave discharges.
In the advanced stages of the disease, a burst suppression and then a diffuse suppression pattern is evident, with eventual isoelectric EEG heralding death.
Atypical prion disease variants in younger populations or related to animal consumption have been described, where the pathognomic CJD pattern on the EEG is absent. Insufficient information exists to comment on the EEG abnormalities in kuru, “mad cow,” and a rare FFI variant of prion disease.
Heidenhain variant of CJD has been described with the predominant occipital abnormalities of the EEG (25).
Generalized sharp wave discharges and multifocal or bifrontal pseudoperiodic or periodic polyphasic discharges with a clinical correlate of a rapidly progressive dementia should alert the practitioner to the possibility of a prion disease; however, other infectious or vascular or toxic-metabolic etiologies should always be considered. Typical diffusion-weighted MRI changes may be of help, but the diagnosis is made on a combination of longitudinal EEG and clinical findings.
Subacute Sclerosing Panencephalitis SSPE is almost an irrelevant condition in the Western world, where measles vaccinations are routine. However, given an active antivaccination minority movement in the developed countries, and the fact that measles is endemic in some underdeveloped countries, it is important to include SSPE in this discussion.
SSPE occurs as a late complication in children who survived a measles infection, and clinically resembles CJD, with rapid intellectual decline, anorexia, spasticity, immobility, myoclonus, choreiform movements, and seizures.
Typical periodic giant complexes are present in the EEG, with the frequent clinical accompaniment of jerks or wholebody spasms.
The pattern, initially described by Radermecker and Poser (26), consists of high-voltage repetitive polyphasic and sharpand slow-wave complexes 0.5 to 2 seconds in duration, occurring multiple times in 1 minute.
Multiple typical and atypical electrographic presentations of SSPE have been described (27, 28, 29, 30 and 31). The typical pattern described by Radermecker, correlated with historical and clinical findings, is definitive for the diagnosis, while atypical EEG patterns of status epilepticus, bifrontal spikes, or other generalized abnormalities as described above may also be useful to the diagnosis.
Encephalitis with Nonspecific EEG Patterns
A multitude of infectious and autoimmune inflammatory processes, with the two coexisting, affect the brain parenchyma, causing disease clinically ranging from mild to catastrophic. Common infections in pediatric and adult world, if investigated with a CSF and EEG, reveal abnormalities in both, with diffuse slowing in the EEG roughly correlated with the onset and the resolution of symptoms. There are hundreds of reports of mildto-moderate-to-fatal encephalitis associated with common pathogens such as coxsackie virus, adenovirus, mycoplasma, rickettsia, mumps, rubella, measles, influenza A and B, and many others. None of those have an EEG pattern of significant diagnostic value. Almost all of these pathogens described as capable of causing meningitis actually cause meningoencephalitis. Bacterial, viral, tuberculous, spirochetal, parasitic, or fungal encephalitis typically has generalized severe polymorphic slowing in the delta range on the EEG, but focal slowing is also possible in granulomatous or parasitic disease.
Viral encephalitis caused by enteroviridae, arboviridae, and echoviridae, with vectors ranging from ticks to mosquitoes, with frequently undetermined etiology, results in EEG abnormalities ranging from mild to severe slowing to electrocerebral silence, and from lateralized potentially epileptogenic discharges to seizures and status epilepticus with burst suppression pattern or with constant seizures (Fig. 18.6). The acute EEG is rarely highly prognostic unless it contains a pattern of burst suppression, which is always ominous, and the long-term prognosis, even with fair for survival, puts the patient at lifelong risk of intractable multifocal seizures. Bilateral mesial temporal sclerosis is not an uncommon complication of viral encephalitis with or without an identified pathogen (Fig. 18.7).
Borrelia burgdorferi-related encephalitis has gotten some recent attention (32,33), although it is not different than other
secondary autoimmune CNS inflammatory processes, and is usually mild and self-limited clinically, with nonspecific encephalopathic changes in the EEG infrequently noted during the course of the disease.
secondary autoimmune CNS inflammatory processes, and is usually mild and self-limited clinically, with nonspecific encephalopathic changes in the EEG infrequently noted during the course of the disease.
Hepatitis, particularly hepatitis A, may cause CNS inflammation and an encephalitic presentation with EEG features of diffuse or focal slowing. West Nile encephalitis generally presents with widespread electrographic abnormalities similar to other viral meningoencephalitis (34), though anterior or temporal predominant slowing has been reported (35). The range of EEG abnormalities is wide, from none to status epilepticus and/or burst suppression pattern that is always ominous (36, 37 and 38).
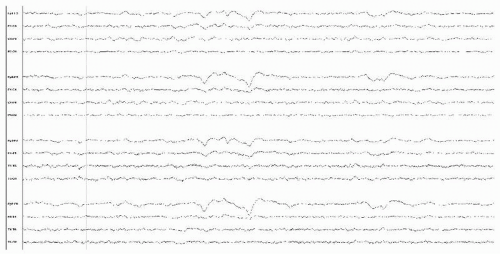 Figure 18.7 This awake-state EEG is from a 60-year-old man with neuroborreliosis, and demonstrates mostly generalized suppression. |
Other encephalitides of viral or presumed viral etiologies also offer no specific EEG patterns of value for diagnosis (39, 40 and 41).
To summarize, specific electroencephalographic diagnosis for most viral or bacterial or fungal or parasitic etiologies of encephalitis does not exist; however, serial EEG recordings provide a good basis for prognostication (42). The amazing thing about viral encephalitis is that a severely abnormal EEG may recover in days or weeks, and the EEG recovery is often correlated with the clinical outcome.
HIV-Related Encephalitis
Clinically or electrographically, AIDS-related encephalitis is not different from other viral encephalitides; however, there is evidence of early CNS involvement and poor prognosis in some studies (43,44). HIV, “the great imitator,” may present with meningoencephalitis at early, middle, or late stages of the disease, without the evidence for secondary opportunistic CNS infection (45). No disease-related or diagnostic EEG pattern specific for HIV meningoencephalitis has been reported, though multiple studies confirmed that a variety of generalized and focal abnormalities in the EEG may be seen, which are different for the acute and chronic phases of the disease (43,44). In the early acute phase, the EEG may be normal or may show the nonspecific pattern of diffuse slowing, while in the late stages, severe delta slowing and even suppression can be seen. Encephalitides caused by opportunistic infections in AIDS, such as Toxoplasma gondii, Cytomegalovirus, and Candida, are discussed above, and may show focal EEG abnormalities corresponding to the underlying lesions, as well as generalized slowing (45).
PART 2: IMMUNE-MEDIATED INFLAMMATORY CNS CONDITIONS
In the conditions discussed in Part 1, the brain is the primary or secondary target of attack by an infection or a presumed infection. In immune-mediated conditions, no CNS infection can be identified, and other organ systems may be the principal site of the inflammation. In cerebral demyelinating disorders and Rasmussen encephalitis the principal site of the inflammation appears to be the brain. In immune-mediated conditions the EEG abnormalities may be diffuse or lesionspecific. In lupus and antiphospholipid antibody diseases, cerebral inflammation is partly secondary to systemic activation of pro-inflammatory cytokines, but the effects on vasculature may also result in ischemia that then accounts for the focal nature of some EEG abnormalities observed clinically. In paraneoplastic CNS injury, although the tumor may be the primary site of immune-mediated attack, the brain is the site of the most damage. Some of the same immunogenic CNS antigens also may be attacked in the absence of a neoplasm, and result in similar damage.
Idiopathic Inflammatory Demyelinating Disorders
Acute disseminated encephalomyelopathy (ADEM) is an umbrella diagnosis for a generally monophasic cerebral demyelination event, with a range of manifestations from mild to very severe. A prodromal event such an upper respiratory infection, recent vaccination, or a flu-like illness is frequently identified, as are recent trauma and surgery. The Marburg variant of fulminant multiple sclerosis (MS), acute hemorrhagic
leukoencephalitis (Hurst disease), tumefactive MS (Balo disease), and Schilder disease in children are related conditions.
leukoencephalitis (Hurst disease), tumefactive MS (Balo disease), and Schilder disease in children are related conditions.
Although white matter is primarily affected in those conditions, the inflammation may affect both normal cortical function and neuronal excitability, and result in EEG abnormalities (46,47) that are varied and nonspecific, with both diffuse and focal delta slowing typical for deep white matter lesions. There may also be focal potentially epileptogenic discharges in tumefactive MS and ADEM (47). With recovery the EEG usually normalizes, somewhat proportional to the extent of recovery from the initial event.
In chronic and late-stage MS, EEG is usually abnormal, with diffuse slowing reflecting the degree of encephalopathy, however, without a clear correlation to radiographic plaque load (48). Although overall only 6.5% of MS patients in a recent study had epilepsy, the percentage was significantly higher in progressive MS patients. The interictal EEG of MS patients with epilepsy may reveal epileptiform abnormalities in up to two third of these patients (49). MS plaques effectively disconnect cortical areas, potentially leading to decreased inhibitory transmission and ictogenesis.
Rasmussen Encephalitis
This progressive epileptic encephalopathy of uncertain etiology and pathogenesis, involving one hemisphere, could be discussed with the rest of the epileptic encephalopathies. These are childhood disorders characterized by cognitive, speech, hemimotor, and functional deterioration, and with progressive EEG changes. However, traditionally Rasmussen disease has been discussed in this section. It is likely that immune-mediated CNS inflammation is part of the etiopathogenesis of other epileptic encephalopathies. In the case of Rasmussen encephalitis, a primary role for the immune attack was proposed (50), but fails to explain the disease adequately (51) as common CNS antigenic responses are seen in many other CNS conditions.
Autoantibodies against glutamate receptors, once thought to be specific for Rasmussen encephalitis, are found in other focal epilepsies, ischemic infarcts, transient ischemic attacks (TIAs), spinocerebellar atrophy, systemic lupus erythematosus (SLE), and paraneoplastic encephalopathies, and are thus not useful in the diagnosis of Rasmussen encephalitis. They are a biomarker for brain injury particular to many encephalopathies, and may be of importance in neurologic dysfunction in various conditions. Immune suppression as a therapy in Rasmussen encephalitis may slow the progression of cerebral degeneration and functional decline, but has limited, and transient, effects on the clinical or electrographic seizures, or on the progression and evolvement of EEG abnormalities (52).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree