The EEG in Patients with Migraine and Other Forms of Headache
Ernst Niedermeyer
Donald L. Schomer
Patients with headaches are usually referred to the electroencephalography (EEG) laboratory to rule out underlying cerebral pathology rather than for a clarification of the type of headache. This type of referral has become less frequent with the greater availability of modern neuroimaging.
Headache is one of the most common complaints. As a symptom, it may herald a wide variety of infectious, neoplastic, and vascular intracranial lesions, but it also may be a sign of various dysfunctions impinging on neural, vascular, and muscular structures. It may arise from the vicinity of the cranial cavity or even from distant structures. Metabolic, toxic, and hormonal disturbances are further causes. In other words, headache is a challenge for the diagnostic acumen of neurologists and other specialists. It has been stated that about 20% of the U.S. population complains of headache, and about half of them receive some form of symptomatic medical treatment (1). Table 24.1 shows a classification of the types of headache. New criteria of classification have been proposed by Silberstein et al. (3).
MIGRAINE (CLASSICAL AND COMPLICATED FORMS)
General Considerations and Clinical Features
Migraine has been known to humanity for ages; a Sumerian poem written 5000 years ago gives an account of this disorder. In spite of a remarkable upsurge of research interest in this field, migraine has remained a poorly understood disorder. A plethora of clinical data is found in the work of Sacks (4).
Table 24.1 Classification of Headache | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
The clinical symptomatology of the migrainous attack is well known. In the classical form, visual symptoms herald the attack; there are scintillating scotoma with teichoscopy and other forms of visual field cuts. Within a short time (about 5 to 20 minutes), this stage is supplanted by headache, which is mostly unilateral, with shifting lateralization from attack to attack in most cases. This is accompanied by severe nausea, vomiting, irritability, and photophobia. This stage lasts for hours or a full day. Wolff (5) has ascribed the initiating visual symptoms to intracerebral local vasoconstriction and the ensuing phase of headache and nausea to an abnormal degree of extracranial vasodilation, which can easily be palpated along the temporal artery on the painful side.
A genetic predisposition is present or even pronounced, but the mode of genetic transmission is not fully understood. The attacks tend to start in adolescence; in childhood, attacks of abdominal pain may be precursors of migraine. It has been thought that a fall of the plasma serotonin level (6) plays a crucial role in the physiopathogenesis of the migrainous attack, but this concept has not been generally accepted. Allergic (antigen-antibody) reactions, free fatty acids, and prostaglandin E are also believed to be involved in migraine (7). Activation of 5-hydroxytryptamine receptors has been stressed by Fozard (8). According to Moskowitz (9), migraine is caused by a disturbance of the “trigeminovascular system” (connections between
trigeminal ganglia and cerebral blood vessels) involving the neurotransmitter peptide, substance P. Experimentally, “neuroinflammation” was produced in animals by electrical stimulation of the trigeminal ganglion causing the release of sensory neuropeptides from nerve terminals (the model of “neurogenic inflammation” (10)). Biggs and Johnson (11) have placed special emphasis on the adrenergic system and its role in migraine pathogenesis. A unified neurogenic concept of migraine has been proposed by Diamond and Dalessio (12).
trigeminal ganglia and cerebral blood vessels) involving the neurotransmitter peptide, substance P. Experimentally, “neuroinflammation” was produced in animals by electrical stimulation of the trigeminal ganglion causing the release of sensory neuropeptides from nerve terminals (the model of “neurogenic inflammation” (10)). Biggs and Johnson (11) have placed special emphasis on the adrenergic system and its role in migraine pathogenesis. A unified neurogenic concept of migraine has been proposed by Diamond and Dalessio (12).
Not all cases of migraine are due to an inherited dysfunction; neuropathologic processes such as arteriovenous malformations of neurosyphilis are known as cases of “symptomatic migraine.” This differential diagnosis may occasionally become difficult, because migraine attacks are capable of proceeding to a state of ischemic infarction (13,14) and of producing regional computed tomography (CT) scan changes (15). In regional cerebral blood flow studies using xenon-133 intra-arterially, reduced blood flow was demonstrable during migraine attacks starting posteriorly and very slowly spreading to the rolandic region (16). According to Hansen et al. (17) and Olesen (18), spreading depression is considered a useful model of migraine aura and presumably also for the subsequent headache. This concept was reemphasized by Olesen (19), especially on the basis of the positron emission tomography (PET) scan observation (oxygen-15-labeled water) of Woods et al. (20). There is good evidence of hypoperfusion within the occipital lobe (20), which can just as well (if not better) be used as supportive of the vascular concept.
Special involvement of the central visual system is a very common feature of classical migraine with visual initiation. Huang et al. (21) have shown with the use of functional magnetic resonance imaging (fMRI) the excessive responses to visual stimuli and a particular sensitivity to a pattern of regularly spaced parallel lines of stripes (see also Lashley (22)).
Whoever reads the original experimental techniques used in the production of spreading depression (23,24) will have nagging doubts concerning the appropriateness of the spreadingdepression model for a neurogenic migraine concept. The mode of elicitation implies all sorts of mechanical and chemical trauma to the brain tissue; electrocorticographic recording (24) shows flattening of the record followed by several recurrent prominent spikes (against a flat background) and another phase of flattening before the baseline character of the record is restored. It is difficult to imagine that similar electrical processes would occur in migrainous human beings. The primordial nature of vasomotor changes according to H. G. Wolff’s (5) original theory seems to be a lot more plausible.
On the basis of data derived from animal experiments (Wistar rats), Ebersberger et al. (25) doubt that spreading depression initiates migraine.
Electroencephalographic Findings
The literature in this field is very confusing because almost equal numbers of reports stress the predominance of normal and abnormal tracings. Relatively few records have been obtained during the attacks; these data are discussed later. Daly and Markand (26) have pointed out that previous studies of migraine and EEG were frequently flawed by sampling problems and heterogeneous populations of migrainous persons.
The contrast between various reports on the EEG in migraine in the interval between attacks is due to (i) composition of material (adults vs. children, inclusion or exclusion of hemiplegic cases), (ii) different criteria for normality and abnormality in the investigators’ EEG interpretation, and (iii) difficulties in the delineation of migraine as a nosologic entity (inclusion or strict exclusion of cluster headaches or symptomatic forms of migraine with cerebral pathology). Keeping all this in mind, it is still difficult to understand the disparity of the reports.
The predominance of normal-interval EEG records was stressed by Ulett et al. (27), Jung (28), Becher (29), Krischek (30), Wissfeld and Neu (31), Bille (32), and Gibbs and Gibbs (33). The work of other authors places the emphasis on a variety of abnormalities. Heyck (34,35) found mainly “hypersynchronous bursts” and occasional focal slowing. Weil (36,37) noted pronounced delta responses to hyperventilation. Various types of abnormalities were noted by Dow and Whitty (38) and Selby and Lance (39). A high incidence of abnormal EEG records was also emphasized by Barolin (40), Gschwend (41), and Pithova (42). Almost equal numbers of normal and abnormal records (with about 45% abnormal tracings) were reported in the extensive work of Smyth and Winter (43). The reported abnormalities, however, were predominantly mild to moderate, with some bursts, slowing, or sharp transients. With the use of computer frequency analysis, Jonkman and Lelieveld (44) demonstrated abnormal interval EEG findings in 55% of migrainous patients. According to Drake et al. (45), the EEG of patients with migraine does not differ significantly from the EEG of normal individuals. This is essentially congruent with my personal views.
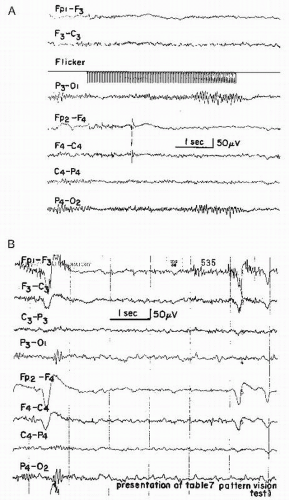
Intermittent photic stimulation often shows an occipital driving response extending into the range above 20 flashes/sec (“H response” after Golla and Winter (46)); according to Smyth and Winter (43), this is almost specific for migraine. This has been substantiated by Slater (47). Personal observations essentially support this view. Further substantiation of these findings has been provided by Simon et al. (48) with the use of spectral analysis during photic stimulation (Fig. 24.1).
EEG findings in the migraine attack range from normal to mildly abnormal (alpha depression) in the initiating ophthalmic phase; even severely abnormal findings have been reported in special cases (49, 50 and 51). Based on his large material, Heyck (34) found normal tracings in the ophthalmic vasoconstrictive as well as in the headache-nausea phase. Schoenen et al. (52) found reduced alpha activity over one occipital region in 19 out of 22 patients recorded during an attack of common migraine. In the light of these observations, the statement that “EEGs have almost always been normal in migraineurs during attacks” (53) might be slightly exaggerated, but EEG abnormalities should be viewed as exceptions.
A neuronal dysfunction as the cause of migraine was assumed by Soysal et al. (54) on the basis of significantly prolonged P100 latencies of visual evoked potentials in the interval between attacks. On the other hand, EEG abnormalities were observed in only 4 of the 13 patients.
When the migraine attack is complicated by mild hemiparetic or dysphasic deficits (migraine accompagnŽe), the EEG may remain normal (55). In cases with pronounced hemiplegia and aphasia, there is good evidence of delta and theta activity over the affected hemisphere (34,56, 57, 58 and 59). The delta activity over the affected hemisphere may be very impressive (see cases of Isler (60)). The neurologic deficit subsides within days (sometimes within weeks), and the focal or lateralized slowing my also linger on for some period of time (Fig. 24.2). Cases of familial hemiplegic migraine have been reported by Whitty (14), Rosenbaum (59), Bradshaw and Parsons (56), and Müller and Müller (61). It has been pointed out that in familial hemiplegia the lateralization of the affected hemisphere remains unchanged in every attack and is the same in all involved family members. This is not congruent with a personal observation. EEG studies in familial hemiplegia show a varying degree of slowing over the brain’s affected side.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree