The Localization of Lesions Affecting the Hypothalamus and Pituitary Gland
Anatomy of the Region
The hypothalamus constitutes the lateral wall of the third ventricle [26,46]. It is separated from the thalamus by the hypothalamic sulcus. The two walls of the third ventricle merge anteriorly to form the lamina terminalis, related superiorly to the anterior commissure and inferiorly to the optic chiasm [22]. Lateroposteriorly, the hypothalamus borders on the globus pallidus, basal forebrain nuclei, internal capsule, subthalamic region, and crus cerebri. An inferior prolongation of the floor of the third ventricle, the pituitary stalk or infundibulum, joins the hypothalamus with the pituitary gland or hypophysis. Each pillar of the fornix, descending rostrocaudally to end in the mammillary body, divides the hypothalamus into a medial and a lateral region.
Main Hypothalamic Nuclear Groups
The hypothalamic nuclei can be conceptualized by considering the hypothalamus as divided by (a) a coronal plane through the infundibular stalk and (b) an angled parasagittal plane containing the fornix. These planes separate four regions: anterior, posterior, medial, and lateral. The topography of the hypothalamic nuclei is illustrated in Figures 17.1 and 17.2.
Connections of the Hypothalamus
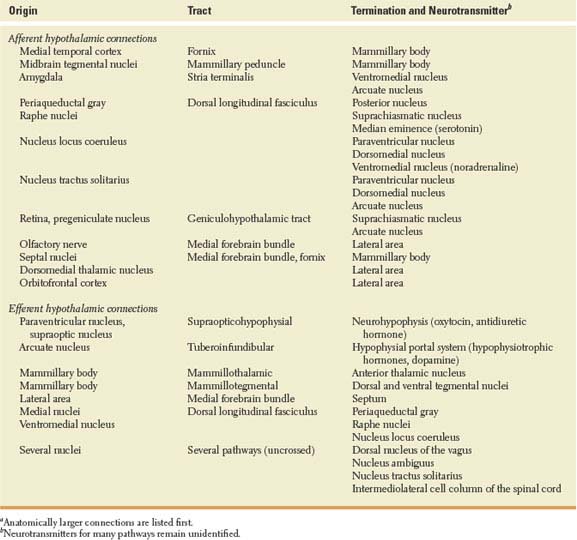
The origin, pathways, and termination of the main afferent and efferent hypothalamic connections are listed in Table 17.1. In summary, the hypothalamus has strong to-and-fro connections with (a) the midbrain and posterior tegmentum, which play an important role in alertness; (b) the limbic system, through the anterior and mesial temporal cortex, anteromedial thalamic region, and amygdala, which play an important role in emotion and memory [122]; and (c) the “autonomic” nuclei of the brainstem and spinal cord, such as the dorsal nucleus of the vagus and the nucleus tractus solitarius. Although direct connections have been traced to the ipsilateral intermediolateral cell column of the spinal cord, much of the influence of the hypothalamus on the autonomic centers of the cord is probably exerted through the brainstem reticular formation [26]. Pathways from the retina and olfactory system convey to the hypothalamus information needed for the circadian control of vegetative functions, and for feeding and reproductive behavior [116].
Much work remains to be done to define the localization and function of the many putative neurotransmitters identified in the hypothalamus [21]. Multiple neuropeptides have been identified in the hypothalamus of experimental animals and humans [49,59,87,100]. In addition to the hypophysiotropic hormones regulating anterior pituitary secretion, other neuropeptides play a role in the regulation of body temperature (bombesin, neurotensin), alertness (orexin, somatostatin), cardiopulmonary function (thyrotropin-releasing hormone, calcitonin gene-related peptide), water balance (enkephalins), circadian rhythms (neuropeptide Y), feeding behavior (cholecystokinin, bombesin, galanin, leptin, neuropeptide Y), and reproductive function (oxytocin, vasoactive intestinal peptide). However, the complex hypothalamic actions of these peptides and others present in high concentration in the hypothalamus (e.g., substance P, motilin, secretin) need to be clarified further [87]. Hypothalamic steroids play an important role in the sexual differentiation of hypothalamic nuclei and in reproductive behavior [21]. Among the biogenic aminergic pathways, best known is the tuberoinfundibular dopamine system, arising in the arcuate nucleus and projecting to the median eminence. Dopamine in the portal system inhibits the release of prolactin [100]. Noradrenergic terminals, originating in the nucleus locus coeruleus and lateral reticular nucleus of the medulla, are found mainly in the paraventricular and retrochiasmatic areas, and in the ventromedial and dorsomedial nuclei. Serotoninergic pathways from the raphe nuclei reach the suprachiasmatic nucleus, suggesting a role for serotonin in the regulation of circadian rhythms. Regarding other neurotransmitters, a cholinergic tuberoinfundibular pathway has been described [97,126].
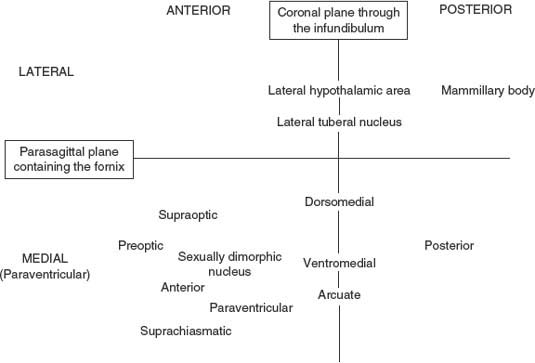
FIG. 17.1. Schematic diagram of the hypothalamic nuclei.
Hypothalamic control of vegetative functions is exerted to a great extent through the pituitary gland. The hormonal secretions of the anterior pituitary are regulated by the hypothalamic-releasing factors or hypophysiotropic hormones, which are released into the infundibular portal system (Fig. 17.3). Through this system, the anterior pituitary receives the richest arteriolar blood flow of any organ in the body, 0.8 mL/g/minute [102]. The infundibulum also contains the important supraopticohypophysial tract, constituted by axons from neurons in the supraoptic and paraventricular nuclei. Those axons end in a rich capillary network in the posterior lobe of the pituitary (neurohypophysis), where they secrete oxytocin and antidiuretic hormone (ADH; also called vasopressin).
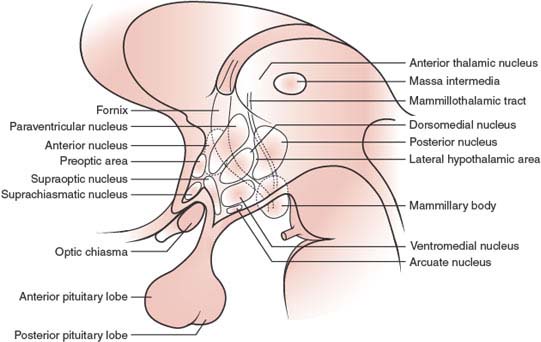
FIG. 17.2. Hypothalamic nuclei.
TABLE 17.1 Connections of the Human Hypothalamusa
Clinical Manifestations of Hypothalamic or Pituitary Dysfunction
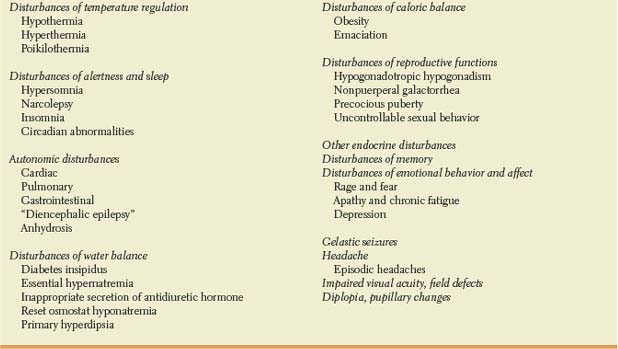
Before discussing the most likely location of a lesion causing such symptoms or signs as are attributable to the hypothalamic-pituitary region (Table 17.2), several points should be noted:
1. Because these structures are small, several portions may be involved simultaneously. For this reason, the fine localization of functions to specific hypothalamic structures is often not known from human lesions and has to be extrapolated from experimental animal data [120].
2. Lesions that progress rapidly cause a more florid clinical symptomatology than those that proceed slowly. For instance, a surgical or vascular lesion in the posterior hypothalamus renders the patient comatose, whereas a slowly growing tumor affecting the same structures causes only apathy.
3. Unilateral lesions are seldom symptomatic.
4. The changes of hypothalamic function with age are reflected in the disparity of syndromes caused in different age groups by similarly located lesions [106]. For instance, a similar lesion may cause dwarfism during childhood and gigantism during adulthood [36].
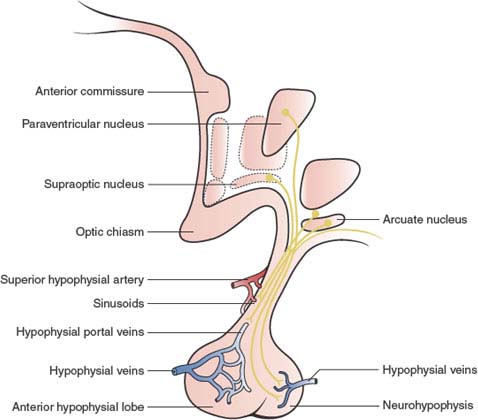
FIG. 17.3. Hypothalamic-pituitary connections.
TABLE 17.2 Clinical Manifestations of Hypothalamic or Pituitary Dysfunction
Disturbances of Temperature Regulation
The hypothalamic “thermostat” for normal temperature regulation is located in the anterior-preoptic hypothalamic area, whose neurons alter their firing rate in response to a warm or cold environment [14]. However, the physiological responses for heating (e.g., vasoconstriction, shivering, increased food intake) and cooling (e.g., increased sweating, peripheral vasodilation, panting, decreased motor behavior) are controlled by mechanisms located in or traversing the posterior hypothalamus. Because heat dissipation is normally needed in a warm ambient temperature, hyperthermia results from anterior hypothalamic lesions, whereas posterior lesions cause hypothermia or poikilothermia by interfering with heat-conservation responses [87,106]. Several neurotransmitters and neuropeptides delivered into the hypothalamus of experimental animals have been shown to induce temperature changes. For instance, serotonin and low doses of opioids induce hyperthermia, whereas high doses of opioids, angiotensin 11, dopamine, acetylcholine, somatostatin, and neurotensin cause hypothermia [87,98]. Their role in temperature regulation in humans needs to be clarified further.
PHYSIOLOGIC RHYTHMS
Diurnal Variation. Body temperature peaks in early evening and reaches the lowest point in early morning [87]. Lesions in the median eminence may flatten the diurnal temperature variation [118].
Menstrual Cycle. Body temperature increases at the time of ovulation; progesterone produces this effect by acting on hypothalamic neurons.
HYPOTHERMIA
Chronic. Most often the lesion involves the posterior or the entire hypothalamus. When discomfort is also present, the lesion may be in the anterior hypothalamus. The most common causes include Wernicke’s encephalopathy [134], head trauma, craniopharyngioma, glioblastoma multiforme, surgery, hydrocephalus, infarction, and sarcoidosis [87].
Paroxysmal. Spontaneous periodic hypothermia; paroxysmal hypothermia with hyperhidrosis; “diencephalic epilepsy.” Rare syndrome characterized by an episodic decrease of body temperature [75,82,96]. The onset is abrupt, with sweating and vasodilatation leading to hypothermia (as low as 30°C rectally) accompanied by nausea, vomiting, hypotension, bradycardia, cardiac arrhythmias, salivation, lacrimation, ataxia, asterixis, and mental dullness. The episodes last from minutes to hours; they may recur only after decades or they may recur more often, even daily. Thermoregulation may be normal between attacks [82]. Responsible lesions have involved the arcuate nucleus and premammillary area. A similar syndrome appeared after surgery for a midbrain glioma [50]. This disturbance may be associated with agenesis of the corpus callosum (Shapiro syndrome) [101] but many patients do not have detectable underlying lesions [14]. One patient had altered norepinephrine metabolism and responded to clonidine therapy [114]. Others have responded to oxybutynin, glycopyrrolate, cyproheptadine, chlorpromazine, or phenytoin suggesting a multiplicity of mechanisms [14].
HYPERTHERMIA
Pyrogen Induced. This is the most common cause of hyperthermia. Cases clinically labeled as “hypothalamic hyperthermia” after an unrevealing search for an infectious source often belong to this category. Bacterial or viral pyrogenes can stimulate directly the hypothalamus and in addition induce the release of interleukin 1 (endogenous pyrogen) from leukocytes and macrophages [37]. Circulating interleukin 1 acts in the preoptic and paraventricular area of the anterior hypothalamus, inducing prostaglandin E2 synthesis, which can be blocked by aspirin [7,87,121]. Sepsis may disrupt hypothalamic function [89].
Acute Hyperthermia. Acute hyperthermia may occur as a consequence of an acute process (craniotomy, trauma, bleeding) and lasts for less than 2 weeks. The lesion affects the anterior hypothalamus. Cardiovascular changes, normally present with fever, are disproportionately small with hyperthermia due to hypothalamic lesions [106].
Paroxysmal Hyperthermia. Involvement of the ventromedial hypothalamus has been suspected in these rare cases, although the precise localization is not known [87,130]. One patient improved after phenytoin administration. Another patient responded to chlorpromazine. This boy had recurrent episodes of fever, hypertension, weight loss, and vomiting, lasting for about 3 days [106]. A patient with agenesis of the corpus callosum and periodic hyperthermia became hypothermic after dopamine administration [61].
Malignant Hyperthermia. Although not due to hypothalamic dysfunction, this syndrome is discussed here with other abnormalities of temperature control. When exposed to various anesthetic agents and other drugs, susceptible individuals may develop a potentially fatal state of severe generalized muscular rigidity, metabolic acidosis, myoglobinuria, and hyperthermia [55,99]. Hyperthermia is thought to be due to an intrinsic abnormality of the excitation-contraction coupling mechanism in skeletal muscle, induced by drug exposure, resulting in sustained myofibrillar contraction. Susceptibility to this syndrome is transmitted as an autosomal dominant trait and in some cases calcium channels are abnormal [54].
Neuroleptic Malignant Syndrome. This syndrome is a potentially life-threatening idiosyncratic reaction to neuroleptic drugs [34,60]. The clinical triad consists of: (a) hyperthermia, usually with other autonomic dysfunctions such as tachycardia, falls in blood pressure, and diaphoresis; (b) extrapyramidal signs, usually increased muscle tone (rigidity) with dystonia, often accompanied by elevated creatinine kinase; and (c) altered mental status, such as inattention, agitation, and confusion. Clinical manifestations usually occur abruptly at therapeutic levels of neuroleptics, with all of the symptoms fully manifest within 24 hours and reaching a maximum within 72 hours. This syndrome, which has also been described following the abrupt withdrawal of levodopa [45,73], can be fatal in up to 20% to 30% of cases. A syndrome with milder fever and a greater tendency to develop myoclonus has been described with the use of serotonin reuptake inhibitors [27].
POIKILOTHERMIA
Poikilothermia is the fluctuation of more than 2°C in body temperature following ambient temperature [2]. It is the most common central neurogenic abnormality of heat regulation in humans. Such patients are unaware of their condition and show no sign of discomfort or behavioral regulation with thermal stress. Poikilothermia results from posterior hypothalamic lesions [106].
Disturbances of Alertness and Sleep
Larger lesions involve the rostroventral components of the ascending reticular activating system. Smaller lesions may cause more localized dysfunction of structures regulating sleep, particularly the suprachiasmatic nucleus, which plays a major role in the regulation of circadian cycles [13], or the hypocretin/orexin nucleus in the posterolateral hypothalamus [9]. The suprachiasmatic nucleus, located in the anterior hypothalamus, receives afferents from the retina and possibly from the lateral geniculate body and projects mainly to other hypothalamic nuclei, but also to the basal forebrain, thalamus, and periaqueductal gray [116]. Circadian rhythms are important for most hypothalamic functions. For instance, oxytocin, which plays a major role in sexual behavior, is secreted mainly in the early hours of the night [44].
COMA, HYPERSOMNIA, OR AKINETIC MUTISM
These are occasionally related to posterior hypothalamic or larger lesions [115]. The most common reported causes have been tumors and Wernicke’s encephalopathy [111,134]. A patient with extreme akinesia after removal of a hypothalamic epidermoid cyst improved with dopamine receptor agonists [112]. Hypersomnia and coma result from midbrain lesions more often than from hypothalamic lesions.
NARCOLEPSY
In the perifornical area of the posterolateral hypothalamus there is a cluster of cells that secrete hypocretin or orexin, a peptide that mediates wakefulness and facilitates feeding behavior [9]. A lack of this substance causes narcolepsy. Given the similar HLA type of the patients, it is speculated that the damage of these cells is of an autoimmune nature [43].
INSOMNIA
Fewer than 10 cases that implicate the anterior hypothalamus have been reported [106]. However, some insomniacs may have increased production of stress-related hormones [133].
CIRCADIAN ABNORMALITIES
Loss of neurons in the suprachiasmatic nucleus occurs in Alzheimer’s disease, attended by phase advance and reduced period and amplitude of the sleep cycles, as well as increased variability and decreased stability of the rhythm [95]. Loss of circadian rhythmicity has also been described with lesions in the region of the suprachiasmatic nucleus, including optic glioma [32,97].
Autonomic Disturbances
Sympathetic areas tend to be ventromedial and posterior. Stimulation of these areas causes hypertension, pupillary dilation, tachycardia, vasoconstriction of vascular beds, vasodilation of muscular beds, and increased cardiac contractility in association with the expression of rage or fear [115]. Hypotension in multiple system atrophy may be in part related to neuronal loss in several hypothalamic nuclei, mainly in the paraventricular nucleus [15].
Parasympathetic areas tend to be paraventricular or lateral, and anterior. Stimulation of these areas causes pupillary constriction. Stimulation of the anterior parasympathetic areas causes hypotension and bradycardia, whereas stimulation of the posterior parasympathetic areas causes only increased blood flow through the bowel and decreased blood flow in skeletal muscle [106]. Although the hypothalamus seems to contribute to the control of micturition in humans, urinary incontinence is not described as a symptom of isolated hypothalamic damage [20].
CARDIAC MANIFESTATIONS
Hypertension, cardiac arrhythmias, electrocardiogram abnormalities simulating myocardial infarction, or even myocardial infarction in a nonvascular pattern may follow subarachnoid or intraventricular hemorrhages, particularly those due to ruptured anterior communicating artery aneurysm [127], but can be observed with other causes of hypothalamic dysfunction, including hydrocephalus [67]. The cardiac damage is mediated by an outpouring of catecholamines. Chronic heart failure with chronic stress may be mediated by the paraventricular nucleus [12].
RESPIRATORY ABNORMALITIES
Pulmonary edema and hemorrhage can result from acute hypothalamic damage (hemorrhage, head trauma). Sudden dysfunction of the parasympathetic region in the anterior hypothalamus, with consequent hypertension, left heart strain, and loss of pulmonary surfactant, may explain the clinical picture [87,106].
GASTROINTESTINAL ABNORMALITIES
Acute hypothalamic lesions (trauma, encephalitis, acute multiple sclerosis, hemorrhage, infarction, abscess, meningitis) can cause gastrointestinal ulceration. Neurogenic ulcers are most often located in the lower esophagus, otherwise an uncommon site for ulceration. Neurogenic ulcers may be caused by acute lesions anywhere in the neuraxis, from the anterior hypothalamic region to the dorsal nucleus of the vagus or even in the spinal cord. Although the hypothalamus is activated during emesis, there is no evidence that hypothalamic damage alters the emetic reflex [64]. Emesis is a prominent feature of the epileptic syndrome in children called “Autonomic seizures and autonomic status epilepticus” or Panayiotopoulos syndrome [103]. In a typical presentation, the child, fully conscious, able to speak and understand, complains “I feel sick,” looks pale, and vomits. Other autonomic symptoms may follow, as well as a generalized seizure. Although the electroencephalogram often shows occipital spikes, Panayiotopoulos is of the opinion that the hypothalamus is involved in the genesis of this syndrome [103].
DIENCEPHALIC EPILEPSY
This term refers to episodes of hypertension, tachycardia, flushing, salivation, sweating, and oscillations in temperature with preserved alertness, but with the behavioral and affective responses appropriate to the altered autonomic response [106]. The electroencephalogram may be abnormal in half the cases, showing slowing but seldom the paroxysmal dysrhythmias characteristic of most forms of epilepsy. About half of the patients have responded to anticonvulsants. Although autonomic disturbances are common in many types of seizures, the clinical picture described above has been found with third ventricular tumors or third ventricular dilation caused by hydrocephalus [87,106].
UNILATERAL ANHIDROSIS OR HYPERHIDROSIS
Unilateral hypothalamic lesions may cause ipsilateral anhidrosis of the body, which is generally incomplete. An ipsilateral Horner syndrome is often present in these cases. Dysfunction of the sympathetic centers in the posterior hypothalamus may be responsible for these findings. Transient hyperhidrosis contralateral to large cerebral infarcts has also been described [79]. No associated autonomic dysfunction was present. In at least one of these cases, the ipsilateral pupil was smaller and the patient was febrile, raising the possibility that the finding may actually represent relative anhidrosis on the side ipsilateral to the infarct. Generalized or segmental hypo- or anhidrosis may be seen with central nervous system conditions such as Shy-Drager syndrome (multisystem atrophy with autonomic failure), Parkinson’s disease, multiple sclerosis, spinal cord disease, stroke, or thalamotomy [29].
Disturbances of Water Balance
Hypothalamic osmoreceptors are in the supraoptic and paraventricular nuclei or their proximity. It has been postulated that intracellular dehydration, manifested by increased intracellular sodium concentration, or extracellular dehydration, manifested by increased angiotensin II concentration in the hypothalamic blood, stimulate these osmoreceptors, which in turn elicit the release of ADH by the large cells of the supraoptic and paraventricular nuclei. By contrast, when the intravascular volume increases, peripheral volume receptors in the large veins and left atrium mediate inhibition of ADH secretion [106].
The lateral hypothalamus, classically considered the drinking center, contains osmoreceptors but may also influence drinking behavior by causing general excitability of the region. In experimental animals, destructive lesions of the lateral hypothalamus cause adipsia (reduced water intake), but not enough to result in dehydration. By contrast, destructive lesions of the ventromedial nuclei may cause hyperdipsia.
DIABETES INSIPIDUS (DECREASED ADH RELEASE BUT NORMAL THIRST)
Although lack of ADH prevents water reabsorption in the distal tubule, with consequent excretion of a large volume of dilute urine, an intact thirst mechanism induces water intake, thereby preventing hypernatremia. Diabetes insipidus [87,106] results from destruction of at least 90% of the large neurons in the supraoptic and paraventricular nuclei. Except for the familial variety, the lesion often involves the supraoptic-hypophysial tract rather than the neuronal bodies themselves. In such cases, the disorder is often transient.
Diabetes insipidus may be familial, linked in some families to a mutation in the vasopressin region of chromosome 20 [72,109], or caused by granulomas (sarcoidosis, meningovascular syphilis, histiocytosis), vascular lesions, trauma, meningoencephalitis, or autoimmune damage to vasopressin-producing cells [85,117]. Anxiety, alcohol, phenytoin, and anticholinergic agents reduce the secretion of ADH.
ESSENTIAL HYPERNATREMIA (DECREASED ADH RELEASE WITH ABSENCE OF THIRST)
Diagnosis of this rare syndrome requires (a) hypernatremia unaccompanied by a corresponding fluid deficiency, (b) preserved renal responsiveness to ADH, (c) impaired secretion of ADH with hypernatremia, and (d) absence of thirst despite preserved conscious behavior [3,56,87,106]. Some patients with the syndrome have a remarkable tolerance to hypernatremia, to the point of developing water intoxication when the condition is treated. Sodium levels reaching 170 mEq per liter, however, are accompanied by muscle cramping, tenderness and weakness, fever, anorexia, paranoia, and lethargy. Lesions causing this syndrome have affected the tuberal region or the entire hypothalamus [41]. The regulation of atrial natriuretic peptide may be abnormal in some patients with a similar metabolic derangement [68]. Other patients have excessive renal responsiveness to ADH [39].
INAPPROPRIATE SECRETION OF ADH (SIADH) (ELEVATED ADH RELEASE WITH NORMAL THIRST)
This syndrome [87,106] is characterized by (a) serum hyposmolarity (<280 mOsm/kg) and hyponatremia (<130 mEq/L), (b) normal renal excretion of sodium, and (c) inappropriately high urine osmolality without body fluid depletion. Renal and adrenal functions are normal. Serum levels of ADH are elevated. The patient has anorexia, nausea, vomiting, and irritability that may progress to paranoid delusions and generalized seizures when the serum sodium falls below 110 mEq per liter. Although SIADH is more often due to extrahypothalamic causes, partial damage of the supraoptic and paraventricular nuclei or neighboring areas may cause the syndrome. Such damage may be due to trauma, subarachnoid hemorrhage, hydrocephalus, tumors, meningitis, encephalitis, or drugs, particularly vincristine, chlorpropamide, cyclophosphamide, carbamazepine, and chlorpromazine. Production of ADH by a tumor (e.g., carcinoma of the lung) or inflammatory tissue outside the hypothalamus may also cause the syndrome, which has also been linked to myxedema, cardiac failure, nonneoplastic pulmonary disease, and to porphyria and other peripheral neuropathies. Impairment of peripheral afferent inhibitory pathways carrying information from the volume receptors to the hypothalamus has been invoked to explain SIADH with polyneuropathies [106]. Often hyponatremia with hypothalamopituitary disease is due to polydipsia with normal ADH levels [124].
RESET OSMOSTAT HYPONATREMIA
Criteria for this diagnosis [6] include normovolemic hypotonic hyponatremia; normal renal, adrenal, and thyroid function; ability to concentrate the urine when serum tonicity is raised above the reset level of serum osmolality; ability to excrete a water load; and maintenance of normal sodium balance without correction of hyponatremia during salt loading.
PRIMARY POLYDIPSIA OR HYPERDIPSIA (EXCESSIVE WATER DRINKING IN THE ABSENCE OF HYPOVOLEMIA OR HYPERNATREMIA)
Patients with this disturbance [106] may drink in response to (a) conditioned behavior (“beer drinker’s hyponatremia,” “tea party epilepsy”) or other psychogenic factors, (b) hyperangiotensinemia, found in renal patients with thirst despite a normal electrolyte balance maintained with hemodialysis, or (c) rarely, hypothalamic disease. In the last case, drinking often compensates for mild diabetes insipidus.
Disturbances of Caloric Balance and Feeding Behavior
The hypothalamus participates in feeding behavior through carefully tuned mechanisms [137]. For instance, neurons in the arcuate and paraventricular nuclei are rich in AMP-activated protein kinase (AMPK), a sensor of the cellular AMP:ATP ratio and therefore a gauge of cellular metabolism [69]. Feeding, hyperglycemia and the anorexigenic hormones insulin and leptin decrease AMPK activity in hypothalamic nuclei. Conversely, fasting, hypoglycemia and the stomach-derived orexigenic hormone, ghrelin, increase hypothalamic AMPK, thus stimulating feeding behavior.
Although disturbances of feeding behavior are not uncommon with hypothalamic lesions, they have been reported also with frontal or temporal lesions, particularly in the right hemisphere [129].
OBESITY
Lesions in the ventromedial portion of the hypothalamus may cause obesity [10]. Characteristically, such patients are hyperphagic (bulimic) until a higher body weight is reached, at which point they maintain a stable body weight unless the lesion progresses. The feeding behavior of patients with hypothalamic lesions resembles that of obese individuals with no demonstrable lesions: Compared to normal individuals, patients with hypothalamic lesions (a) eat only a slight excess of food each day, (b) are less active, (c) eat fewer meals each day, (d) eat more at each meal, (e) eat more quickly, (f) eat more of a good-tasting food, (g) eat more when food is easily accessible, and (h) react more emotionally and are appeased by food intake [87]. Obesity after ventromedial lesions may result from affection of the catecholaminergic pathways coursing in this region, rather than from destruction of the nuclei themselves. Most frequent lesions include craniopharyngioma, pituitary adenoma, surgery for the removal of these tumors, other types of trauma, encephalitis, and vascular lesions of the base of the brain [23,94]. Compulsive eating may be caused by rather nonspecific brain lesions. Occasionally, it may respond to anticonvulsant therapy [53]. Impaired secretion of cholecystokinin has been described in bulimia nervosa [48]. Hypothalamic serotoninergic stimulation reduces food intake, particularly of carbohydrates, and increases energy expenditure, thus reducing weight [81]. The following obesity syndromes are thought to be due to hypothalamic dysfunction.
Kleine-Levin Syndrome. This is a rare variety of compulsive eating behavior in adolescent males, characterized by episodes of hyperphagia, with or without excess appetite, periodic hypersomnolence, hyperactivity when awake, and behavioral disturbances, particularly hypersexuality and exhibitionism. Although traditionally considered a hypothalamic derangement, medial thalamic pathology was reported in one case [28] and hypopigmentation of the substantia nigra and locus coeruleus in another [76]. A viral illness preceded the onset of the syndrome in some cases. The disorder usually disappears during the third decade. Endocrinological evaluation has failed to show abnormalities during the periods of abnormal behavior [90].
Prader-Willi Syndrome. This syndrome comprises obesity, hypogenitalism, mental retardation, short stature, micromelia, and a tendency to develop diabetes mellitus [63]. Affected infants tend to be hypotonic (neonatal hypotonia), somnolent, and eat little, but between 6 months and 2 years of age, they begin to eat in excess and become obese. Abnormal luteinizing hormone-releasing hormone neurons are thought to be responsible for the decreased levels of sex hormones, resulting in nondescended testes, but primary testicular dysfunction may account for undersized sex organs and insufficient growth during puberty [62]. A lack of growth hormone-releasing hormone may also contribute to the short stature of patients with Prader-Willi syndrome [35]. In addition, the aberrant control of body temperature and daytime hypersomnolence may result from hypothalamic disturbances. The number of oxytocin neurons—the putative satiety neurons—in the hypothalamic paraventricular nucleus is markedly decreased in Prader-Willi syndrome. This is presumed to be the basis of the insatiable hunger and obesity of patients with the syndrome [83]. It is caused by a lack of paternal genetic information at 15q11-q13, due to impaired paternal imprinting of several genes [51].
Laurence-Moon-Bardet-Biedl Syndrome. This syndrome comprises obesity, hypogonadism, mental deficiency, retinitis pigmentosa, and polydactyly [52,87]. Diabetes insipidus and renal failure are often present. The condition is transmitted as an autosomal recessive trait, genetically heterogenous, with at least four loci located to date. In most families the defect is linked to 11q13 [11]. Hypothalamic lesions have not been found [135].
EMACIATION
Diencephalic Syndrome of Infancy. This syndrome is a distinct clinical condition characterized by emaciation, with loss of subcutaneous fat, pallor, motor overactivity, and an inappropriately jovial behavior [87,106]. Progressive emaciation occurs despite normal food intake. Nystagmus, optic atrophy, and tremor are less frequently encountered manifestations. Growth hormone levels may be high. The syndrome usually appears in boys aged 3 to 12 months and is caused by a slow-growing astrocytoma of the anterior hypothalamus or optic nerve. Children that survive beyond their second year become obese and irritable.
Lateral Hypothalamic Syndrome. Few case reports deal with lateral hypothalamic lesions causing aphagia, cachexia, and death [106]. Multiple sclerosis [70], tumors [5], and trauma have been implicated. Weight loss in Huntington’s chorea has been ascribed to neuronal loss in the lateral tuberal nucleus [77]. An increased cortisol secretion has also been documented in this disorder [8].
Anorexia Nervosa. This is characterized by anorexia, weight loss, and amenorrhea in an otherwise endocrinologically normal young woman [30]. Although the syndrome suggests hypothalamic dysfunction, in most cases no morphologic changes have been found in the hypothalamus [92].
Disturbances of Reproductive Functions
Hypothalamic lesions frequently alter reproductive functions both by decreasing gonadotropin substances that exert a trophic effect on sexual organs and by altering the neural mechanisms of intercourse. For instance, the paraventricular nucleus plays an important role in penile erection [4]. Several hypothalamic nuclei, particularly the GABA-containing sexually dimorphic or intermediate nucleus, have been found in young adults to be twice as large in men as in women, but the function of these nuclei is still unclear [47].
HYPOGONADOTROPIC HYPOGONADISM
This type of hypogonadism may follow any hypothalamic or pituitary lesion [87,136]. It is manifested by amenorrhea or male gonadal dysfunction. When no lesion is found, the condition in women is termed functional hypothalamic amenorrhea, which has an endocrinologic pattern closely resembling the findings in depression (decreased reproductive hormones, increased growth hormone and cortisol) [16,93]. Hypothalamic hypogonadism has been documented in individuals subjected to extreme exercise programs, such as marathon runners [84]. More frequent reproductive dysfunction in women with epilepsy may reflect hypothalamic-pituitary changes due to the disease and the effect of anticonvulsant medications [66].
NONPUERPERAL GALACTORRHEA
Prolactin-Secreting Pituitary Tumors. About one-third of chromophobe adenomas secrete prolactin.
Structural Damage to the Infundibulum or Hypothalamus. Such damage may interrupt the dopaminergic pathway that inhibits prolactin secretion by the pituitary. This pathway originates in the arcuate nucleus [87].
Other Causes. Other causes of nonpuerperal galactorrhea include irritative lesions of the anterior chest wall, thoracic spinal cord lesions, neuroleptic and contraceptive drugs, and hypothyroidism [87].
PRECOCIOUS PUBERTY
Although generally idiopathic [40], precocious puberty may be due to hypothalamic disease or pineal tumors, some of the latter affecting also the floor of the third ventricle [24,110].
EXCESSIVE OR UNCONTROLLABLE SEXUAL BEHAVIOR
Occasionally, this behavior may be a consequence of lesions of the caudal hypothalamus [107].
Other Endocrine Disturbances
Disorders such as panhypopituitarism, hypothalamic hypothyroidism, acromegaly, and Cushing’s syndrome are reviewed in standard endocrinology textbooks. In neonates, it is important to recognize and treat the panhypopituitarism of congenital aplasia of the pituitary because treatment is lifesaving [119].
Disturbances of Memory
The mammillary bodies are frequently involved in Korsakoff’s amnestic syndrome and in experimental animals they seem to play an important role in memory mechanisms [131]. However, when alcoholics with and without amnesia are studied, amnesia correlates with neuronal loss in the anterior nucleus of the thalamus, not in the mammillary bodies [57]. Other dorsal hypothalamic lesions have been accompanied by amnesia [10,71]. Bilateral interruption of the mamillothalamic tract is considered by some to be critical for the production of memory loss by lesions in the ventromedial hypothalamus [74,86,108].
Disturbances of Emotional Behavior and Affect
RAGE AND FEAR: INAPPROPRIATELY DISINHIBITED BEHAVIOR
When caused by hypothalamic lesions, rage and fear occur in episodic outbursts, usually triggered by a threatening or frustrating event (such as restraint or a delay in feeding) and are part of a fully coordinated behavioral response with an intense autonomic component [78,104,106]. Between the outbursts, the behavior is normal and the patient may realize the inappropriateness of such behavior and apologize for it. Attacks of rage may also result from lesions of the orbitofrontal cortex, septal region, or temporal lobe [1]. When the hypothalamus is responsible, the ventromedial region is usually involved. By contrast, stimulation of the posterior “sympathetic” area elicits responses of fear and horror [115]. Some patients with hypothalamic lesions have the uninhibited behavior thought to be characteristic of orbitofrontal lesions [88]. Hypothalamic hormones play a role in modulating social interactions. Oxytocin secretion, for instance, seems to enhance trusting on other people [123].
APATHY, CHRONIC FATIGUE
Apathy may follow lesions of the posterior or lateral hypothalamus. Bilateral stereotactic lesions in this area have been used to treat unmanageable aggressive behavior due to brain pathology [115]. Although some cases of the chronic fatigue syndrome have been chalked to dysfunction of the hypothalamopituitary-adrenal axis, including mild hypocortisolism, this axis is normal in patients studied early in the process [31].
DEPRESSION
Although there is no evidence that depression is caused by hypothalamic dysfunction, many depressed persons have an excessively active hypothalamic-pituitary-adrenal axis [139]. Postmortem studies after suicides have shown increased numbers of corticotropin-releasing hormone (CRH) neurons and increased vasopressin expression in the paraventricular nucleus, increased CRH in the CSF and decreased CRH receptors in the frontal cortex. Challenge with dexamethasone and corticotropin-releasing hormone is the most sensitive test available to detect altered hypothalamic-pituitary-adrenal system regulation in depression [105].
Gelastic Seizures
Seizures accompanied by involuntary laughter (gelastic seizures) have been described in association with precocious puberty [24]. Hypothalamic hamartoma is most often the cause and origin of the seizures [78,128]. The patient may stare and giggle briefly, without any other motor manifestation. Crying or sobbing seizures may alternate with the gelastic spells in the same patient. Other patients only have an interior feeling of pressure to laugh, without overt manifestations [125]. Hypothalamic hamartomas often cause executive function disorders (“frontal lobe syndrome”) [78,104]. Gelastic seizures have also been described with cortical dysplasia in the cingulate gyrus [91].
Headache
In one out of seven patients with a pituitary tumor, the presenting complaint is headache. This is usually bitemporal or bifrontal, behind the eyes, and is thought to be due to compression of the diaphragma sella [87]. Sudden worsening of headache, particularly of a retro-orbital nature, in a patient with a pituitary tumor should raise the diagnostic suspicion of pituitary apoplexy, discussed below. Headache is more frequent with pituitary abscesses, a rare condition, than with tumors [132].
EPISODIC HEADACHES
Increased perfusion in the posterior-medial hypothalamus, dorsal to the mammillary bodies, has been documented with PET during some episodes of cluster headache, paroxysmal hemicrania or the type of headache known as “short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT)” [113]. Deep-brain stimulation of this area has resulted in the improvement of patients with these disorders [42].
Chronic Pain
The hypothalamic-pituitary-adrenal axis seems to be dysfunctional in many patients with chronic pain syndromes, such as fibromyalgia [19].
Impaired Visual Acuity; Visual Field Defects
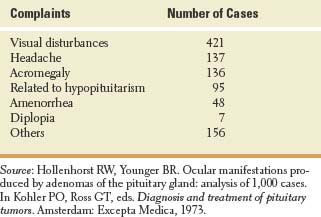
Some patients with pituitary adenoma present with visual complaints, and about 15 % or more have decreased visual acuity or field defects on formal testing (Table 17.3). Bitemporal defects, related to compression of the inferior aspect of the chiasm, are most common. However, asymmetric tumor growth may cause preferential involvement of one eye, with unilateral blindness, or of the optic tract, with consequent homonymous hemianopia. The position of the chiasm in relation to the sella also determines the pattern of the visual field defect. The chiasm lies over the sella in 80% of brains, over the tuberculum sellae in 9% of brains (prefixed chiasm), and over the dorsum sellae in 11% of brains (postfixed chiasm) [17]. About 6% of patients have central or temporal scotomas, which may pass unnoticed if only the periphery of the visual field is tested.
TABLE 17.3 Presenting Complaints in 1,000 Cases of Pituitary Adenoma