2. Nonsteroidal anti-inflammatories (NSAIDs). NSAIDs exert their analgesic effect via inhibition of the cyclooxygenase pathways and include naproxen, ibuprofen, tolfenamic acid, and diclofenac. Naproxen has the longest half-life allowing it to be taken twice daily. Relatively higher doses (e.g., naproxen 500 to 1,000 mg, ibuprofen 800 mg) may be needed. The NSAIDs share a similar side effect profile, with gastrointestinal symptoms, such as dyspepsia and nausea, being the most common. NSAIDs are contraindicated in patients who are anticoagulated or have peptic ulcer disease, and may be contraindicated for patients with severe renal impairment, ongoing alcohol abuse, severe gastroesophageal reflux disease, or a history of gastric bypass or Nissen fundoplication. There is increasing evidence that long-term use of NSAIDs may increase the risk of heart attack or stroke.
3. Combination analgesics. Various combinations of NSAIDs, acetaminophen, caffeine, and aspirin are available and should be restricted to less than 10 days per month.
4. Headache-specific treatments. These medications are commonly referred to as “migraine-specific” therapy even though they can be effective for cluster headache. They are employed if simple analgesics and NSAIDs are not sufficiently effective at aborting attacks. In addition, various combinations of nonspecific and headache-specific analgesics are available both with and without a prescription. ICHD-3b defines overuse of these medications as 10 or more days per month.
a. Ergot alkaloids. In the 19th century, ergotamine, and later in the 20th century, dihydroergotamine, were the first medications to be used specifically for acute migraine treatment. Both have a wide spectrum of pharmacologic activity at serotonergic, adrenergic, and dopaminergic receptors. Although effective, these medications are burdened by side effects, the most significant of which are nausea, vomiting, and vasoconstriction with a subsequent labeled contraindication for patients with coronary or cerebrovascular disease. Of historical note, the efficacy of ergotamine derivatives was attributed to their vasoconstrictor effects and the triptans were developed to retain this property with fewer side effects. Nowadays, ergotamine derivatives are not as commonly used as the better-tolerated triptan class. Ergotamine is available in oral, sublingual, and rectal formulations. Dihydroergotamine has intramuscular, intravenous, and intranasal formulations; in addition, an orally inhaled formulation is in late-stage development.
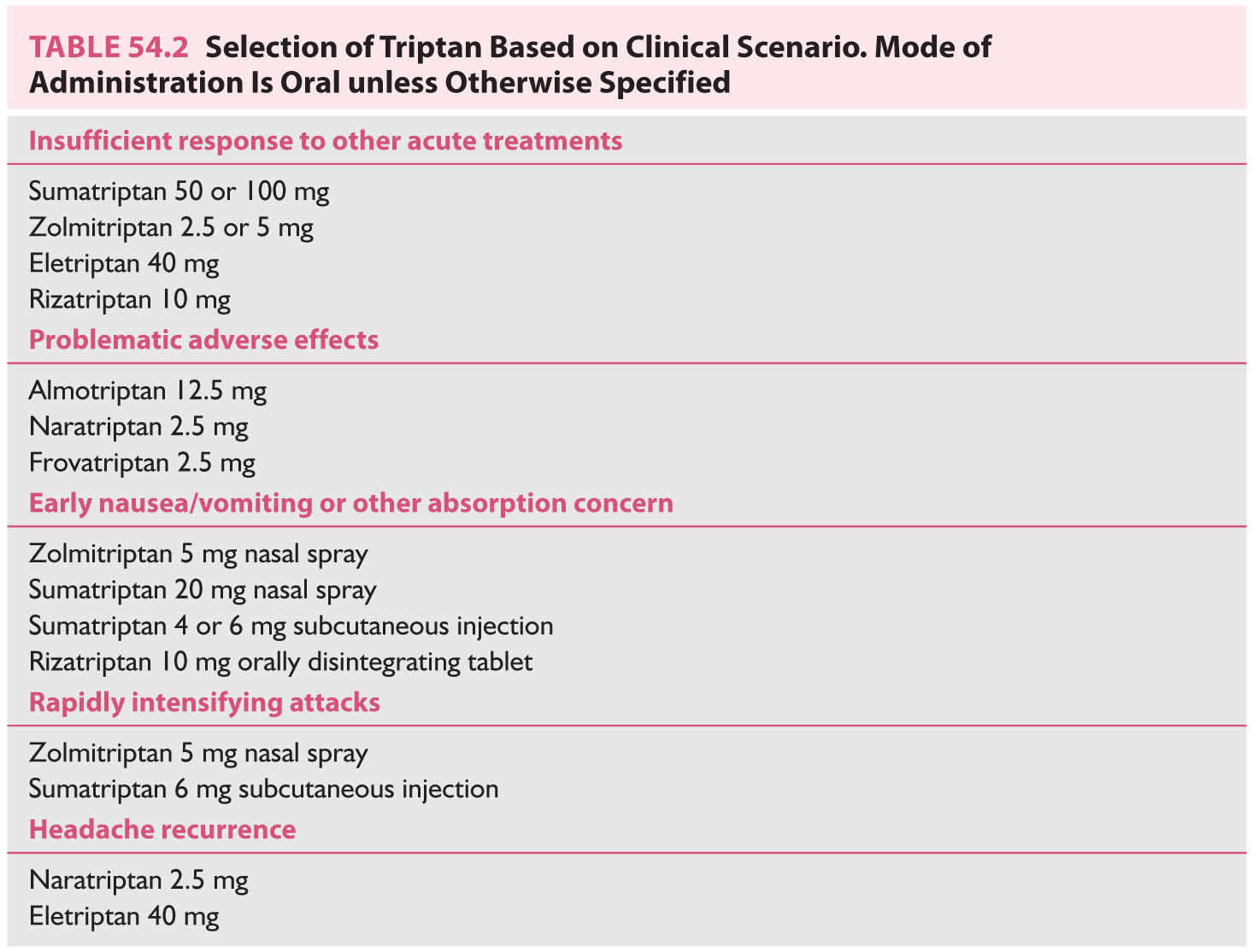
b. Triptans. Triptans are serotonin 5-HT1B/1D receptor agonists. Sumatriptan first became available in 1991; six additional triptans are now available that differ in their pharmacokinetic and side-effect profiles, which can be used to clinical benefit (Table 54.2). In addition, sumatriptan and zolmitriptan are available as a nasal spray and sumatriptan as an injection, which can benefit patients with significant nausea, vomiting, or attacks that intensify rapidly. In terms of efficacy, most triptans achieve a 2-hour pain freedom rate in the range of 25% to 40%. Common side effects include tingling paresthesias and warm sensations in the head, neck, chest, and limbs. Less common but perhaps more concerning to patients are chest-related symptoms- chest pressure, which can radiate into the arm, shortness of breath, palpitations, anxiety, or drowsiness. Triptans have vasoconstrictor actions mediated by their effects on the 5-HT1B receptor, and thus also have a labeled contraindication for patients with coronary or cerebrovascular disease and for use within 24 hours of other vasoconstrictors such as dihydroergotamine. However, triptans are currently considered to exert their antimigraine effect via neuronal mechanisms, and in line with this, agents selectively active at the 5-HT1F receptors (the “ditans”) are being developed and have efficacy against migraine without vascular effects.
5. Other abortive approaches. A range of other approaches are used in Emergency Departments, or indeed in outpatients, for the treatment of acute migraine. Chlorpromazine has been used and there is some evidence for this treatment. Corticosteroids offer no better pain relief than placebo when they have been studied systematically. Nerve block procedures are used, including greater occipital nerve (GON) injection and other cranial nerve injections, although there is no systematic well-controlled evidence supporting the practice.
6. In development: CGRP mechanism antagonists. This is the first class of agents (the “gepants”) to target a migraine-specific mechanism. CGRP is a peptide formed from alternative splicing of the calcitonin gene. CGRP is released into the jugular venous system after activation of the trigeminovascular system during migraine attacks. Serum CGRP levels are also elevated interictally in chronic migraine and to a lesser extent in episodic migraine. Finally, infusion of CGRP can trigger migraine-like attacks in migraineurs. Six CGRP receptor antagonists have been through at least phase II studies and each is reported to be more effective than placebo. Telcagepant and MK-3207 have had their programs terminated due to hepatic toxicity, while olcegepant has not been progressed as it is not orally bioavailable. Rimagepant and BI44370TA are not currently being developed, while ubrogepant (MK-1604) was effective in a phase II study and has moved to phase III studies. No data exist regarding the effects of overuse of this class of agents, although since telcagepant has been shown to be effective also as a preventive, it is entirely possible that medication overuse will not be a class issue. Supporting this conclusion, biologics targeting the same mechanism with long-lasting antagonism reduce attack frequency (see Section B under Pharmacotherapy). Importantly, CGRP receptor antagonists do not have vasoconstrictor effects and will not be contraindicated for patients with cerebral or coronary vascular disease.
7. Opioids and barbiturates. Barbiturates have no useful place in modern migraine therapy. Opioids may have limited uses in particular settings over the short term. These medications present several issues: first, they are the most prone to causing medication-overuse headache, particularly barbiturates. Second, they may render both acute and preventive therapy less effective. Third, frequent use often engenders tolerance or dependence, requiring escalating doses over time. Last, withdrawal of the offending substance in a patient taking high doses may not be possible as an outpatient, and access to an inpatient withdrawal program may be limited. In the case of opioids, clonidine may be useful for mitigating withdrawal symptoms. Barbiturates carry the additional risk of withdrawal seizures and may require a supervised taper to be safely discontinued.
8. Antiemetics. Nausea is often overlooked and is an important symptom to treat if present. Phenothiazine antiemetics (D2 dopamine receptor antagonists), such as prochlorperazine and chlorpromazine, have been shown in placebo-controlled trials to also be effective for headache. Possible adverse effects shared by most anti-dopaminergic medications include drowsiness, akathisia, dystonia, and prolongation of the QT interval at higher doses. Serotonin (5-HT3 receptor) antagonists, such as ondansetron or granisetron, may be better tolerated. For patients with significant vomiting, sublingual dissolving tablets or suppositories may be useful.
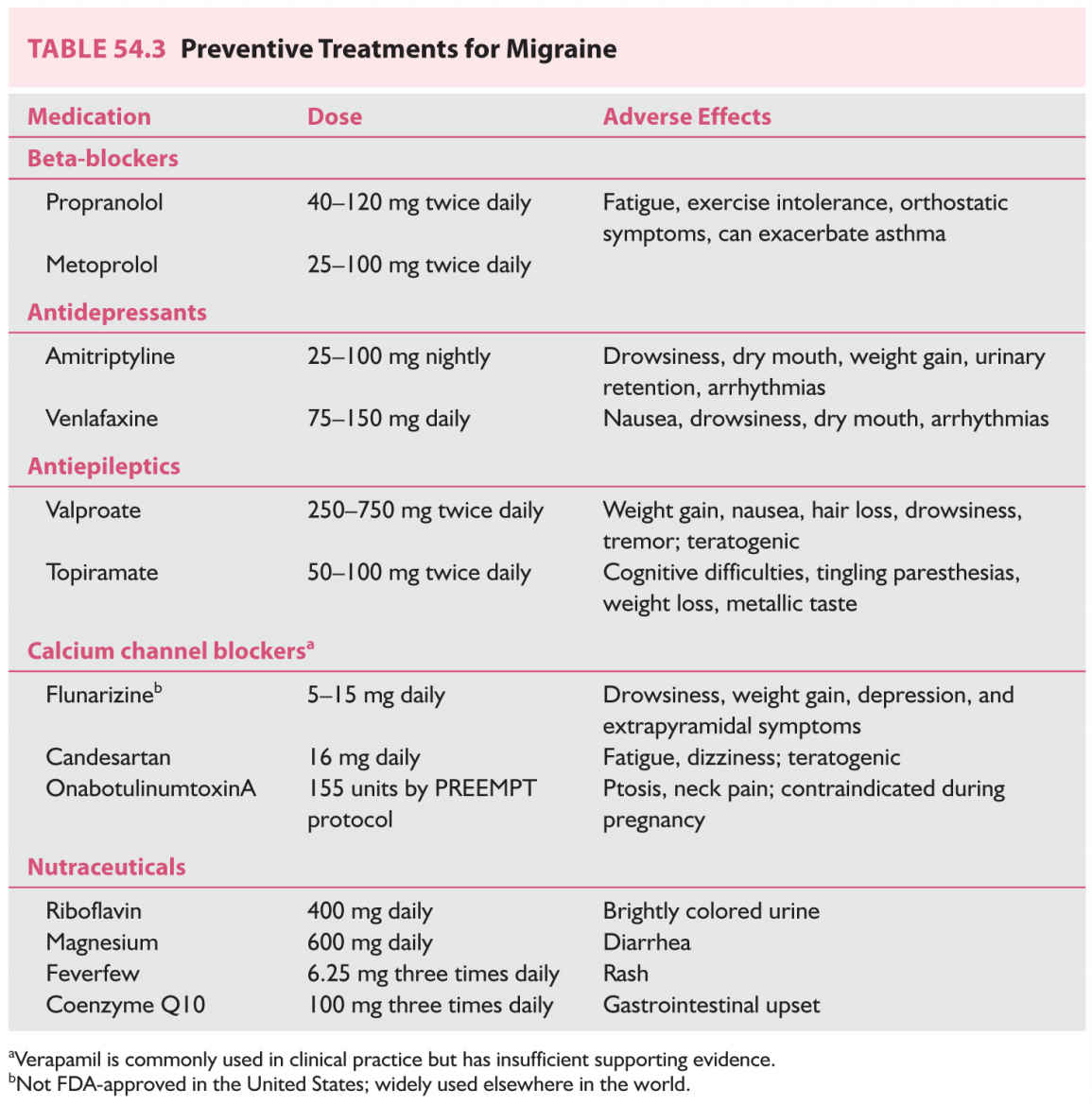
B. Preventive treatments (Table 54.3). Preventive therapies are meant to reduce headache frequency and must be taken daily, except for onabotulinumtoxinA, which is injected every 3 months. The decision to initiate preventive therapy is individualized and may depend on several factors in addition to attack frequency, such as the severity and disability associated with attacks, their duration and responsiveness to acute therapies, and the patient’s willingness and ability to comply with the preventive medication regimen. Preventive treatment should certainly be considered if abortive use is increasing and especially if bordering on medication overuse. An important principle in migraine prevention is that an adequate dose is reached and maintained for at least 6 to 8 weeks before judging the efficacy. Currently, migraine preventives comprise a diverse group of medicines developed for other diseases; the arrival of the previously discussed antagonists targeting the migraine-specific CGRP receptor mechanism will be a welcome addition.
1. β-Adrenoceptor antagonists. Multiple agents in this class have randomized, double-blinded, controlled clinical trial data to support their use for migraine prevention. Propranolol and metoprolol are perhaps the most commonly used, although timolol, atenolol, and nadolol may be used as well. One small trial suggests that nebivolol may be effective. Beta-blockers should be avoided in patients with asthma, and used with good discussion in athletes.
2. Antidepressants.
a. Tricyclic antidepressants. Amitriptyline is a serotonin and norepinephrine reuptake inhibitor and was first shown to be effective for migraine prevention in the 1970s. The tricyclic antidepressants also antagonize histamine and muscarinic receptors; side effects include drowsiness, dry mouth, and weight gain. Nortriptyline is a metabolite of amitriptyline with different receptor binding affinities and is often better tolerated. Caution should be used in the presence of other serotonin-promoting medications (e.g., selective serotonin reuptake inhibitors) to minimize the risk of serotonin syndrome.
b. Venlafaxine. Venlafaxine is a selective serotonin and norephinephrine reuptake inhibitor (SNRI). Two clinical trials, one placebo-controlled and one crossover comparator study with amitriptyline, support its use for migraine prevention. The most common side effect in both trials was nausea.
c. Other antidepressants. Other antidepressants are sometimes used to treat migraine, but this clinical practice is not evidence-based as most have not been formally studied in blinded, randomized, controlled trials. Multiple trials of fluoxetine for migraine prevention have yielded mixed results and many are of poor quality; the overarching outcome is a lack of effect. Fluvoxamine has no placebo-controlled data but one study comparing it to amitriptyline found comparable efficacy. One placebo-controlled, crossover study supports the use of trazodone for prevention of pediatric migraine but no controlled data exist for adults. Other antidepressants including citalopram, escitalopram, and duloxetine only have uncontrolled, open-label data.
3. Antiepileptic drugs.
a. Valproate. Both divalproex and sodium valproate have been shown to be effective for migraine prevention. Studies suggest that efficacy is comparable to propranolol or amitriptyline. Side effects can be troublesome and include weight gain, nausea, hair loss, drowsiness, and less commonly tremor. Valproate is teratogenic (neural tube defects in particular) and should only be used in women of reproductive age if a reliable form of contraception is used. Caution should be used in pediatric populations with underlying metabolic or mitochondrial disorders as hepatoxicity has been reported.
b. Topiramate. Multiple large randomized, controlled, double-blinded trials have shown topiramate to be effective for prevention of both episodic and chronic migraine. Topiramate’s mechanism of action against migraine is not fully understood, but it inhibits excitatory glutamatergic activity, enhances GABA receptor type A-mediated inhibition, and inhibits cortical spreading depression in animal models. Side effects include cognitive difficulties, tingling paresthesias, metallic taste, and in contrast to other migraine preventives, may suppress appetite and cause weight loss. Caution should be used in patients with a history of nephrolithiasis or glaucoma.
c. Gabapentin. In the past, gabapentin was recommended for migraine prevention based on two placebo-controlled trials. However, full disclosure of the studies demonstrated they were both negative. In addition, a large, randomized, controlled trial of gabapentin enacarbil, a prodrug that is rapidly converted to gabapentin after absorption, was reported in 2013 and was negative. The case of gabapentin emphasizes the need for transparency of data from industry-sponsored trials and careful diligence by investigators to see the data from a study that is being reported.
4. Calcium channel blockers.
a. Flunarizine. Flunarizine is a selective calcium channel antagonist with a long half-life of approximately 18 days. Although numerous randomized, placebo-controlled clinical trials have shown flunarizine to be effective for migraine prevention and at least as effective as propranolol, metoprolol, and pizotifen (5-HT2 serotonin antagonist), it is not FDA-approved for use in the United States. It is widely used for migraine prevention elsewhere in the world. Side effects include drowsiness, weight gain, depression, and extrapyramidal symptoms with long-term use, likely owing to its antidopaminergic properties. It is particularly useful in hemiplegic migraine.
b. Verapamil. Although verapamil is commonly used in clinical practice for migraine prevention, only two randomized, controlled trials exist and each included less than 15 patients. More data are needed to support or refute its use in migraine. Side effects include constipation and ankle edema.
5. Candesartan. Two randomized, double-blinded, controlled clinical trials have shown candesartan to be effective for migraine prevention, with efficacy comparable to propranolol. Side effects include fatigue and dizziness.
6. OnabotulinumtoxinA. Multiple clinical trials have shown onabotulinumtoxinA injections are not effective for episodic migraine. Two clinical trials (PREEMPT 1 and 2) demonstrated efficacy for chronic migraine, and only this group should be treated with onabotulinumtoxinA using the protocol employed in these trials. The PREEMPT protocol consists of 31 injection sites in the head and neck totaling 155 units and is generally very well tolerated. Side effects include forehead weakness and possible ptosis or neck pain. Use is contraindicated during pregnancy.
7. Nutraceuticals. A number of naturally occurring supplements such as vitamins, minerals, and herbal extracts have been studied for migraine prevention. Two double-blinded, randomized, controlled trials have shown efficacy for butterbur, an extract from Petasites hybridus. However, this plant also contains hepatoxic elements (pyrrolizidine alkaloids) that pose serious safety concerns. Riboflavin 400 mg daily, magnesium 600 mg daily, feverfew 6.25 mg three times daily, and coenzyme Q10 100 mg three times daily may be used for migraine prevention; although much of the clinical trial data have significant methodologic limitations, each of these has at least one positive double-blind, randomized, controlled trial. Nutraceuticals may be useful for patients who are hesitant to take prescription medications or are particularly prone to side effects.
8. GON block. The GON carries sensory information for most of the posterior aspect of the head and is derived primarily from the C2 dorsal root. GON blocks have been employed in the treatment of various headache types although its mechanism is unknown. Upper cervical and trigeminal afferents converge onto second-order neurons in the trigeminocervical complex which then project rostrally from the brainstem, and the effect of GON blocks may be mediated by modulation of this activity. The existing literature on GON blockade for migraine is difficult to interpret. Most studies are open-label and uncontrolled, and patient blinding is problematic since nerve blocks typically result in hypoesthesia in the affected distribution. Many studies have significant methodologic flaws, and in addition heterogeneous outcome measures are used, with some studies examining acute pain relief at 20 minutes and others examining reduction of frequency or severity at weeks to months. Likewise, methods vary considerably including the use of repetitive blocks versus single injections, the addition of corticosteroids to anesthetic or not, and unilateral versus bilateral injections. Despite the lack of definitive evidence supporting the use of GON blocks for migraine, many clinicians and patients feel they are useful and they are commonly utilized in clinical practice.
9. In development: CGRP mechanism antagonists. Inhibition of this mechanism (see Section A under Pharmacotherapy for more detail) is being targeted for migraine prevention in addition to acute treatment. Currently there are four biologics, monoclonal antibodies, in development, three aimed at CGRP itself and one at the CGRP (CLR/RAMP1) receptor and one small molecule CGRP receptor antagonist (atogepant). Initial results have been promising, and the success of these agents in phase III trials would mark the beginning of a new era in migraine therapy with the first migraine-specific target. Long-term safety data are needed, as CGRP may have important nonmigraine functions that are not yet known or understood.
C. Inpatient therapies. For the most refractory patients who do not respond satisfactorily to outpatient treatments, admission for a course of intravenous therapy may be useful. The literature on inpatient treatments is uncontrolled and observational; the most evidence exists for dihydroergotamine and lidocaine.
1. Dihydroergotamine (DHE). Repetitive dosing of intravenous DHE every 8 hours for 3 days for intractable migraine was first described by Raskin in 1986. Headache-freedom was achieved within 48 hours for 89% of 55 patients who had continuous headache for at least 2 months, with most reporting lasting benefit at follow-up. Two large, retrospective studies have since supported the efficacy and safety of DHE used in this manner, although one study was confounded by concurrent use of corticosteroids and nonsteroidal anti-inflammatories. The other study extended DHE administration to 5 days finding that higher total DHE dose produced a better outcome. Two studies show that good nausea control improves the outcome from DHE. Nausea is the most common side effect of DHE, requiring premedication with antiemetics.
2. Lidocaine. Two retrospective reviews have reported improvement in chronic daily headache, mostly migraine, with intravenous lidocaine infusion for 7 to 10 days. In one study, the majority of patients reported benefit lasting at least 6 months. The other study was confounded by concurrent use of intravenous DHE or corticosteroids as needed. Side effects include nausea, hypotension, psychiatric side effects, hallucinations, and arrhythmia.
3. Valproate. One uncontrolled study of ten chronic migraine patients found that repetitive dosing of intravenous valproate every 8 hours for 2 to 7 days led to improvement in eight patients.
4. Lysine acetylsalicylate (aspirin). For patients using high doses of acute medications such as opioids or barbiturates, admission may be necessary to provide a supervised environment in which to discontinue the overused agents with aggressive management of withdrawal symptoms. Cessation of the overused acute medications often results in worsened headache that is in itself intolerable to the patient, and for this intravenous aspirin may be useful. One gram of intravenous aspirin, given primarily for medication withdrawal headache in an inpatient setting, was moderately effective or better in 89% of 91 migraine patients and was well tolerated, even in patients with a history of upper gastrointestinal problems or NSAID intolerance.
NEUROMODULATION
For many patients, current pharmacologic therapies offer insufficient efficacy and a high rate of adverse effects. Neuromodulation is increasingly being explored for both acute and preventive treatment of migraine. Peripheral nerve stimulation has long been used to treat other types of chronic pain and was the first approach applied to migraine. Evaluation of such devices is plagued by blinding difficulties because nerve stimulation is perceptible and may produce paresthesias or pain. Transcranial magnetic stimulation (TMS) offers another approach to neuromodulation by using a fluctuating magnetic field to induce an electrical current in the underlying cortex which can either inhibit or potentiate cortical excitability depending on the frequency and pattern of stimulation used.
A. Peripheral nerve or ganglion stimulation. The mechanism by which peripheral neurostimulation impacts migraine is not known but may be related to the convergence of upper cervical and trigeminal afferents onto second-order neurons in the medullary trigeminocervical complex. These neurons then project rostrally in the brainstem and to the diencephalon, and are anatomic pathways by which peripheral afferent input might modulate central processes. The occipital nerves (upper cervical roots) and supraorbital nerves (ophthalmic division of trigeminal nerve) have been targeted for neuromodulation with modest results. The sphenopalatine ganglion (SPG) and vagus nerve are under investigation as new potential targets.
1. Occipital nerve stimulation. The multicenter ONSTIM trial examined occipital nerve stimulation using implanted leads as a preventive treatment for chronic migraine. The primary outcome was 50% reduction of pain severity, and there was no significant difference between the 105 patients randomized to active stimulation and the 52 sham stimulation patients. In addition, there was a high rate of device-related adverse events, with lead migration in 19% of patients and persistent pain or numbness at the lead site in 22%. The PRISM trial, available only in abstract form, and a third trial also did not show a statistically significant difference in primary outcome between groups.
2. Supraorbital neurostimulation. In the PREMICE trial, noninvasive supraorbital transcutaneous stimulation for 20 minutes daily with the Cefaly device reduced the number of monthly migraine days after 3 months of treatment when compared to a sham stimulation group. The treatment is remarkably well tolerated, with only 4.3% of over 2,300 users reporting minor adverse events and 2% discontinuing use.
3. SPG stimulation. The sphenopalatine gangnalion (SPG) is an extracranial ganglion located in the pterygopalatine fossa. Its postganglionic parasympathetic fibers innervate the lacrimal and salivary glands and blood vessels of the nasal mucosa, playing a central role in the cranial autonomic symptoms seen in both migraine and the trigeminal autonomic cephalalgias (TACs). Reversible blockade of this ganglion via electrical stimulation was first studied for cluster headache and appeared to have both acute and preventive effects, leading to exploration of its use in migraine. In a small pilot study, stimulation of the SPG with a temporary lead aborted or relieved migraine headache in 5 of 11 patients. An exploratory study in three patients with episodic migraine has gathered preliminary safety and efficacy data on an implanted SPG stimulator for both acute and preventive treatment of migraine (ClinicalTrials.gov NCT01294046), and a trial for prevention in chronic migraine is underway (NCT01540799). A slightly different approach to SPG blockade with twice weekly, trans-nasal pharmacologic blocks did not find statistically significant differences between bupivacaine and saline groups within 2 hours of treatments or after 6 weeks.
4. Vagal nerve stimulation. Anecdotal reports of migraine improvement in epileptic patients treated with implanted vagal nerve stimulators led to two open-label, pilot studies for acute treatment of migraine using a noninvasive device held against the neck (GammaCore). Both have shown some efficacy for acute migraine treatment, and a preliminary trial investigating this device for chronic migraine prevention has been completed and was reported as negative in an abstract (NCT01667250). Adverse effects include paresthesias or redness at the site of device application, neck twitching, or raspy voice.
B. Transcranial magnetic stimulation. Two single-magnetic pulses delivered 30 seconds apart to the back of the head with the Cerena Transcranial Magnetic Stimulator at aura onset resulted in a higher rate of pain freedom at 2 hours (39% vs. 22% in the sham group) and sustained pain freedom at 48 hours (27% vs. 13% in the sham group). The Food and Drug Administration (FDA) approved this device for acute treatment of migraine with aura in December 2013 on the basis of this study. A similar but smaller product, the Spring TMS, is available for clinical use in the United Kingdom. The device is very well tolerated, with transient lightheadedness being the most common adverse effect reported in a postmarketing survey. Repetitive TMS is under investigation for prevention of chronic migraine (NCT02122744).
SURGICAL TREATMENT
Surgical procedures targeting extracranial nerves of the head and face, for example, branches of the trigeminal nerve or greater or lesser occipital nerves, are sometimes advertised to migraine patients for prevention. Options include nerve “decompression” via removal of overlying muscle or the nerve sheath, or even radiofrequency ablation or resection. Although reports of benefit have been published, no true randomized, double-blind, placebo-controlled trials of such procedures exist. Given the irreversible nature of many treatments and potential risks of anesthesia, such an approach does not seem warranted.
TENSION-TYPE HEADACHE THERAPY
The ICHD-3β defines tension-type headache as the absence of the features used to define migraine headache, requiring two of the following four: bilateral (vs. unilateral for migraine), nonthrobbing (vs. throbbing), mild or moderate intensity (vs. moderate or severe), and absence of movement sensitivity (vs. presence for migraine). Photophobia or phonophobia but not both may be present, and mild nausea is permitted only if photophobia and phonophobia are absent; the presence of either photophobia with phonophobia or nausea defines migraine. Appendix criteria define tension-type headache as having none of nausea, photophobia, or phonophobia; this definition sharpens the distinction and is recommended for clinical practice. Like migraine, tension-type headache is categorized as episodic (14 or fewer days per month) or chronic (15 or more days per month), with episodic tension-type headache further subdivided into infrequent (less than 1 day per month) or frequent forms.
In clinical practice, migraineurs often report also having headaches that phenotypically meet criteria for tension-type headache, and this is reflected in clinical trials for migraine which frequently include both migraine days and total headache days as separate outcomes. Ascribing two distinct biologic entities to explain the observed range of headaches, rather than viewing migraine and tension-type headaches as lying on a continuum in terms of severity and associated features, seems unnecessarily complicated. Notably, many migraine treatments are also effective for tension-type headache.
Patients with infrequent or low-frequency episodic tension-type headache often do not come to medical attention, as their symptoms can typically be managed with nonprescription analgesics. For patients with chronic tension-type headache, treatments that have randomized, double-blinded, controlled trials to support their use include amitriptyline, sodium valproate, stress management, and acupuncture (for prevention). If these are not effective, use of proven migraine treatments is a logical approach.
CLUSTER HEADACHE THERAPY
Cluster headache is one of the trigeminal autonomic cephalalgias (TACs), a group of primary headache disorders characterized by lateralized attacks of varying duration that may be associated with particularly prominent, ipsilateral cranial autonomic symptoms, such as conjunctival injection, lacrimation, nasal congestion or rhinorrhea, or ptosis. The TACs also share imaging properties featuring activation of the posterior hypothalamic region. Cluster headache is characterized by attacks of excruciating, unilateral pain typically lasting from 45 to 90 minutes but with a wider range of 15 to 180 minutes allowed, with attacks occurring from every other day up to several times per day. For most cluster headache patients, attacks are not always present; the periods during which attacks occur are referred to as cluster periods or bouts, with bouts typically lasting for weeks to months. The ICHD-3b defines episodic cluster headache as having at least 1 month annually without attacks; if attacks are present continuously or remissions are shorter than 1 month, the condition is chronic cluster headache. Periodicity is a hallmark feature of the disorder, with attacks frequently displaying a circadian pattern and, in episodic cluster headache, bouts often exhibiting a circannual pattern (Video 54.1). ![]()
PHARMACOTHERAPY
Similar to migraine pharmacotherapy, cluster headache treatments fall into two categories: acute treatment of attacks as they occur and preventive therapy which has the primary goal of ending the bout by suppressing attacks.
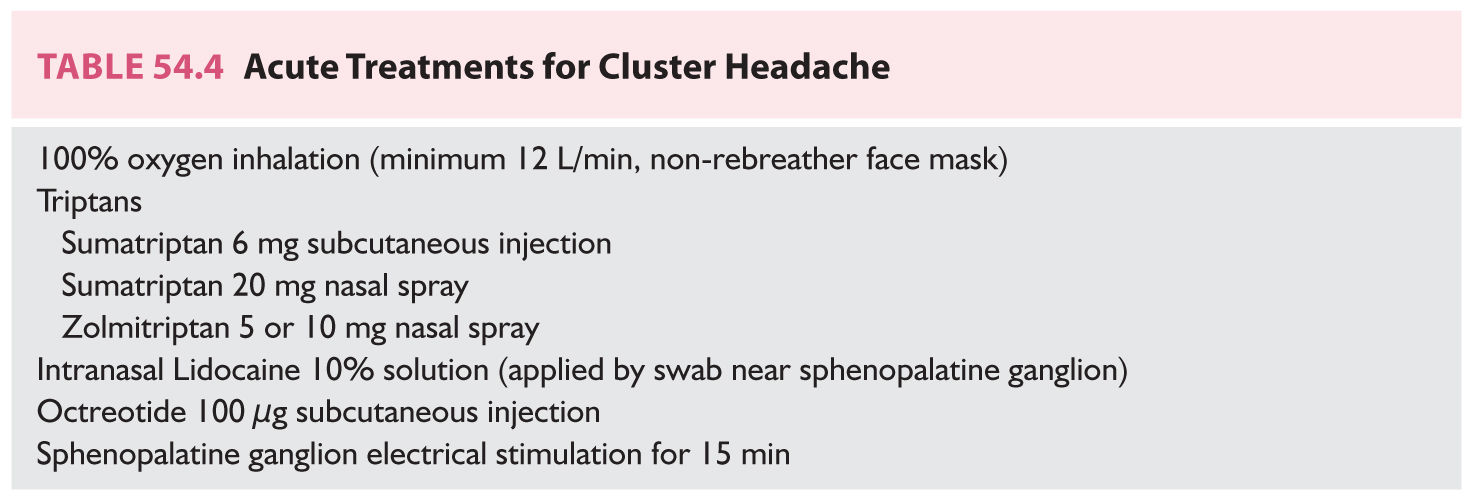
A. Acute/abortive treatments (Table 54.4). In general, the severity of cluster headache attacks intensifies too rapidly for oral formulations to be useful or practical. Inhaled or injectable treatments form the mainstay of acute cluster headache therapy, with high-flow oxygen and triptans being the primary treatments. In contrast to migraine, it is not clear whether frequent abortive use increases the frequency of cluster headache attacks. Conflicting reports describe chronic subcutaneous sumatriptan use both with and without a subsequent increase in attack frequency; some were confounded by the withdrawal of prophylactic agents immediately prior. In a retrospective review of 430 cluster headache patients, 4% described chronic daily headache associated with medication overuse, nearly all of whom had a personal or family history of migraine. In addition, tachyphylaxis does not appear to be of significant concern for acute cluster headache treatment.
1. High-flow oxygen inhalation. Although reports of oxygen inhalation to treat cluster headache attacks had existed for decades, it was not systematically studied until 1981, albeit in an unblinded fashion. Delivery of 100% oxygen at 7 L/minute by face mask for 15 minutes aborted attacks in 75% of patients. A crossover comparison with sublingual ergotamine showed comparable efficacy and faster onset of relief with oxygen. A small, double-blinded, crossover study of pure oxygen versus compressed room air corroborated oxygen’s abortive effect, which was later confirmed by a large randomized, double-blinded, crossover study of 100% oxygen at 12 L/minute by non-rebreather face mask for 15 minutes. A small double-blinded, crossover study showed no difference between pure oxygen and normoxic air given under hyperbaric conditions. High-flow oxygen can be used repetitively without adverse effects and is relatively free of medical contraindications given the short duration of use, which is of particular benefit to patients with several attacks per day.
2. Triptans. Sumatriptan and zolmitriptan have randomized, double-blinded, placebo-controlled data showing efficacy for acute treatment in both episodic and chronic cluster headache. The efficacy of subcutaneous injection of sumatriptan 6 mg was reported in 1991, which offered considerable convenience and portability advantages over oxygen tanks. Subsequently, both sumatriptan 20 mg nasal spray and zolmitriptan 5 and 10 mg nasal spray were shown to be effective, offering cluster headache patients additional options with cost and tolerability advantages. Zolmitriptan 10 mg tablet was also shown to be effective for aborting cluster attacks but only met the primary endpoint in patients with episodic cluster headache. Patients are generally advised to limit triptan use to no more than twice daily given their vasoconstrictor effects.
3. Ergot derivatives. Only one placebo-controlled, crossover trial has examined intranasal dihydroergotamine (DHE) 1 mg for acute treatment of cluster headache. It found that DHE reduced the intensity but not duration of attacks. Of note, this trial used a smaller dose than is typically used for migraine (4 mg). Other outpatient delivery methods of DHE have not been formally studied for cluster headache. Historically, oral, sublingual, inhaled, or intravenous formulations of ergotamine were used to treat cluster headache, but reports of their efficacy were open label and uncontrolled. Given the medical contraindications and side effects, ergotamine has generally been abandoned in favor of treatments with better evidence and tolerability.
4. Intranasal lidocaine. Two open-label series in the 1980s reported that lidocaine 4% solution applied as drops intranasally, targeting the pterygopalatine fossa, was effective at aborting nitroglycerin-induced and spontaneous cluster attacks. A larger series using sprays of lidocaine solution had more modest results. A small double-blinded study comparing lidocaine 10% solution to saline, applied by nasal swab targeting the pterygopalatine fossa, found lidocaine to be significantly more effective but required a mean of 37 minutes to fully abort an attack.
5. Subcutaneous octreotide. During a cluster attack, calcitonin gene-related peptide and vasoactive intestinal polypeptide are released; somatostatin is an endogenous peptide that inhibits their release. Subcutaneous injection of octreotide, a somatostatin analog with a longer half-life, was shown in a double-blinded, two-attack crossover study to be more effective than placebo for acute treatment of cluster headache.
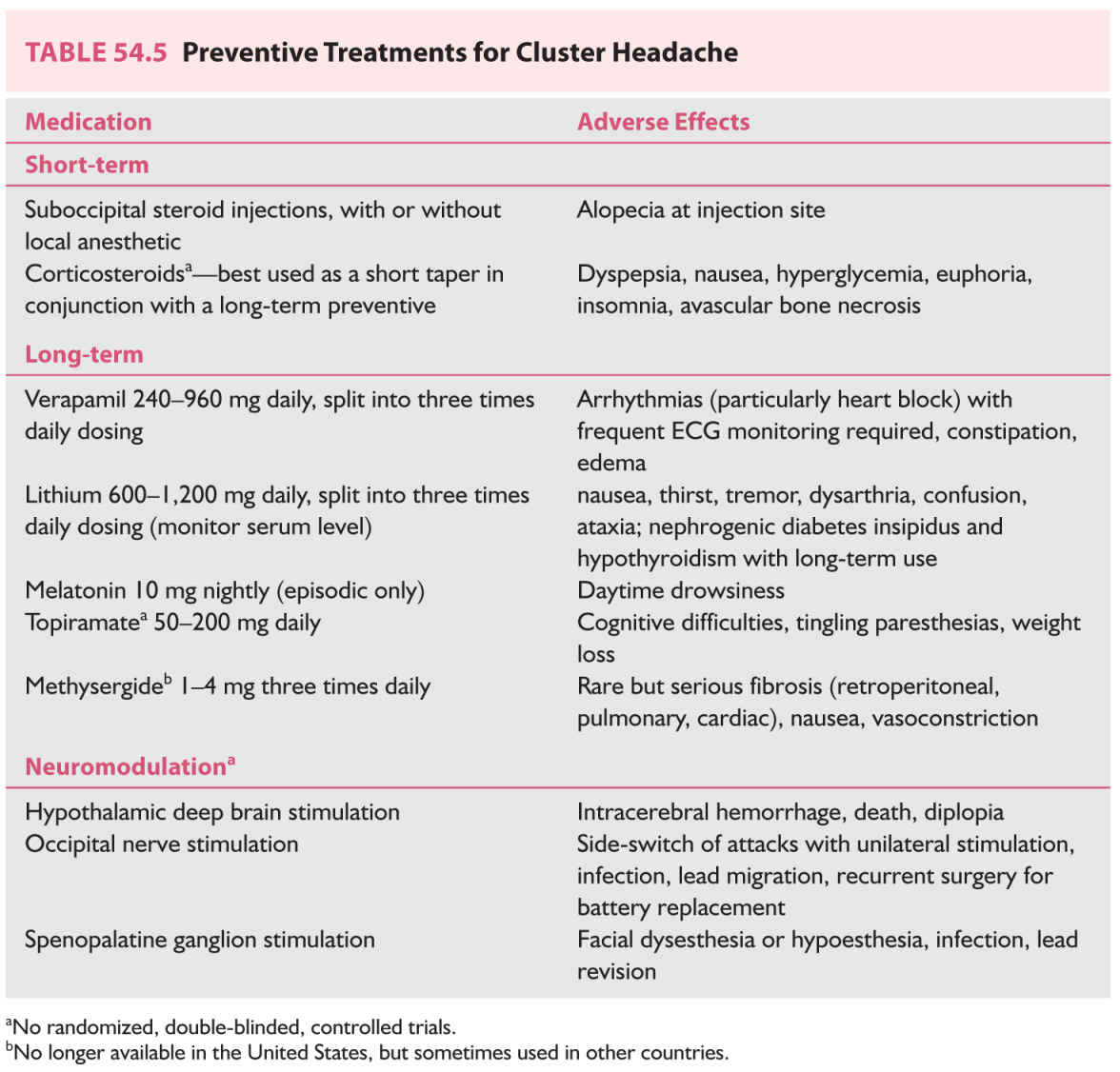
B. Preventive/prophylactic treatments (Table 54.5). Preventive treatment for cluster headache has the goal of suppressing attacks and either terminating the bout or reducing the frequency of attacks. Verapamil, the first-line cluster headache preventive therapy, requires a slow titration and thus other short-term preventive treatments may be used in combination.
1. Short-term prevention.
a. GON block. The preventive effect of GON block in cluster headache was first reported by Anthony in 1985. Subsequent open-label studies of GON block with steroids, some with the addition of local anesthetic, have had variable outcomes. Two randomized, double-blinded, controlled studies including both episodic and chronic cluster headache patients have examined suboccipital steroid injections for cluster headache prophylaxis. The first study compared betamethasone with a small amount (0.5 mL) of lidocaine to saline with lidocaine and found that attacks ceased within 5 days for 12 of 13 patients in the steroid group; 9 of 13 remained attack-free 4 weeks after injection. In contrast, 0 of 10 patients injected with saline and anesthetic were attack-free 1 week and 4 weeks after treatment. The second study compared a total of three injections of cortivazol, a potent and long-acting steroid, versus saline when given every 2 to 3 days as add-on preventive therapy, most commonly in conjunction with verapamil. The baseline attack frequency was higher in this study, and it showed a significantly greater reduction in mean attack frequency and sumatriptan usage for 15 days after treatment in the steroid group.
b. Corticosteroids. High-quality evidence supporting the use of corticosteroids in cluster headache is lacking. Several open-label and uncontrolled studies suggested an effect but had wide variability in the dose, formulation, and duration of corticosteroid use. Some studies have demonstrated a recurrence of attacks upon discontinuation of steroids in as little as 2 days, and corticosteroids are necessarily for short-term use only given their multiple adverse effects with prolonged use. Thus, steroids are best used in conjunction with a long-term preventive therapy to provide initial relief. A randomized, double-blinded, placebo-controlled trial of prednisone as add-on therapy to verapamil for episodic cluster headache is underway in Germany (PredCH).
2. Long-term prevention.
a. Verapamil. Open-label reports on the efficacy of verapamil for cluster headache prophylaxis led to two randomized, double-blinded trials. A crossover study in chronic cluster headache patients comparing 8 weeks of treatment with verapamil 120 mg three times daily or lithium 300 mg three times daily, the standard of care at the time, with 2-week washout (placebo) periods, found both to be efficacious in reducing attack frequency and analgesic use. Verapamil had a shorter latency period and fewer side effects. A parallel-group study in episodic cluster headache showed verapamil 120 mg three times daily for 2 weeks to be more effective than placebo in reducing attack frequency. Verapamil is the first-line preventive therapy for cluster headache, and the dose may need to be increased to a maximum of 960 mg daily to achieve full effect. Electrocardiographic (ECG) abnormalities are the primary safety concern, and in a retrospective review of 108 cluster headache patients who had taken verapamil, arrhythmias were present in 19%. First-degree heart block was the most common finding (62% of the abnormalities), but second-degree heart block, junctional rhythm, and right bundle branch block were also seen. Patients with arrhythmias were not taking higher doses of verapamil than those without. In another retrospective study of 29 patients taking verapamil 720 mg daily or higher, 14% presented with first-, second-, or third-degree heart block. Both series reported cases of heart block appearing years after reaching a stable verapamil dose. Thus, patients on verapamil therapy must be closely monitored with ECGs throughout the duration of therapy. Other common side effects include fatigue, constipation, and ankle edema.
b. Lithium. Lithium’s use in cluster headache was inspired by its efficacy in other diseases with a marked cyclical nature such as bipolar disorder, and it was first reported to be effective for three cluster headache patients by Ekbom in 1974. Numerous open-label case series subsequently corroborated its effect, suggesting in sum that lithium is more effective for chronic cluster headache than episodic, that its therapeutic effect can begin within days and likewise withdrawal of the medication can result in rapid worsening, and that its effect may wane with long-term use although tolerance was an inconsistent finding. Lithium has been studied in two randomized, double-blinded studies including the aforementioned crossover study with verapamil in chronic cluster headache patients showing comparable efficacy. The other study attempted to compare 1 week of slow-release lithium 800 mg daily to placebo in episodic cluster headache patients, but assumed zero placebo response which proved to be untrue, rendering the study underpowered for drawing any conclusions. Lithium 600 to 1,200 mg daily can be used for cluster headache prevention and is typically titrated to a serum concentration between 0.4 and 0.8 mEq/L or to cessation of attacks. Lithium has a narrow therapeutic window and possible side effects include nausea, thirst, tremor, dysarthria, confusion, and ataxia. Renal and thyroid function tests prior to treatment are necessary as long-term use may cause nephrogenic diabetes insipidus and hypothyroidism.
c. Melatonin. One small, randomized, double-blinded, placebo-controlled trial of melatonin 10 mg nightly suggested that melatonin may reduce attack frequency in patients with episodic cluster headache.
d. Topiramate. All studies examining topiramate for cluster headache prevention have been open-label and uncontrolled. While initial reports suggested a response, the largest series of 36 patients found no difference in attack frequency after 20 days of treatment.
e. Methysergide. Methysergide is a serotonin antagonist that is no longer available in the United States due to its risk of fibrosis with long-term use, primarily retroperitoneal although pulmonary and cardiac effects have also been seen. In other countries, it is still sometimes used for cluster headache not responsive to other preventives; to minimize risk of fibrosis, a 1-month drug holiday should be taken every 6 months.
f. Intranasal capsaicin and civamide. Two studies have reported on intranasal capsaicin for prevention of cluster headache. One reported a decrease in severity but not frequency of attacks in the week following 1 week of treatment, and the other reported a decrease in attack frequency for 2 months following intranasal capsaicin ipsilateral but not contralateral to the side of pain. Intranasal civamide, a synthetic isomer of capsaicin, was found to reduce attack frequency compared to vehicle placebo, but only in the first week after treatment. These treatments are not recommended for routine use.
C. Inpatient treatment. Two series have reported improvement in cluster headache attack frequency after repetitive dosing of intravenous dihydroergotamine (DHE) every 8 hours for 2 to 6 days, with headache-freedom achieved during treatment in nearly all patients. In both series, improvement in attack frequency lasted for months in a subset of patients.
NEUROMODULATION
A. Deep brain stimulation (DBS). Hypothalamic DBS was first reported to be effective at reducing attack frequency in a case of chronic cluster headache in 2001. The posterior hypothalamus was chosen as a target based on functional and structural imaging studies showing alterations in this region and the clinical features of circadian and circannual periodicity, functions attributed to the hypothalamus. A number of open-label series subsequently supported the long-term preventive efficacy of hypothalamic DBS in refractory chronic cluster headache patients. However, a randomized, double-blinded, crossover study did not find a difference in attack frequency between active and sham stimulation periods; this may have been due to a short treatment period. Nonetheless, when also considering that one patient in Belgium died from intracerebral hemorrhage along the electrode track, DBS has largely been abandoned in favor of less invasive approaches.
B. Occipital nerve stimulation. Occipital nerve stimulation has been explored as a safer neuromodulation option for prevention of cluster headache. Several series have reported improvement in medically refractory chronic cluster headache patients, with two-thirds of patients in the worldwide literature experiencing at least 50% reduction in attack frequency. All have been open-label and uncontrolled, and blinding presents considerable methodologic difficulty since effective occipital nerve stimulation produces paresthesias. The therapeutic effect takes weeks or months to appear and can be long-lasting, although attacks recur within days if the stimulation is discontinued. Bilateral lead implantation is recommended as side-shift of attacks can occur with unilateral treatment. The main adverse effects are related to hardware, including immediate or delayed infection requiring lead or battery explantation, lead migration, or additional surgery for battery replacement.
C. SPG stimulation. The SPG sits extracranially in the pterygopalatine fossa and its postganglionic, parasympathetic fibers serve as the major outflow pathway for the cranial autonomic symptoms that are a clinical hallmark of the TACs. It has been targeted with pharmacologic blocks with transient results. Radiofrequency ablation of the SPG in chronic cluster headache patients resulted in sustained improvement in frequency and intensity of attacks. Nondestructive functional inhibition can be achieved with electrical stimulation. A small series examining SPG stimulation with a temporary lead for acute treatment of both spontaneous and triggered cluster headache attacks resulted in complete relief within 3 minutes for 11 of 18 attacks, with at least 50% relief in another three attacks. A multicenter, randomized, sham-controlled, multiple-attack study (Pathway CH-1) examined an implanted, remote control-activated SPG microstimulator as an acute treatment in chronic cluster headache patients. Pain relief after 15 minutes of treatment was achieved in 67% of attacks, with pain freedom achieved in 34% of attacks, compared with 7% and 2% of attacks, respectively, after sham stimulation. In addition, an unexpected preventive effect was seen, with 12 of 28 patients experiencing at least 50% reduction in attack frequency. A similar study is underway in a larger population of chronic cluster headache patients (Pathway CH-2, ClinicalTrials.gov NCT02168764). SPG stimulation is minimally invasive, relatively safe, and its potential as an acute treatment without daily usage limits that has concurrent preventive effects is an exciting development for a population in dire need of additional treatment options.
D. Vagus nerve stimulation. A preliminary, open-label, observational study of a noninvasive vagus nerve stimulator has suggested benefit for both acute and preventive treatment of cluster headache, warranting further study with randomized, controlled trials.
SURGICAL TREATMENT
Surgical treatments for chronic cluster headache patients comprise a range of approaches to disrupt or destroy either trigeminal sensory or cranial parasympathetic fibers. These include glycerol application, radiofrequency ablation, or gamma knife surgery to the trigeminal ganglion or nerve or SPG. With trigeminal procedures affecting the V1 distribution, corneal anesthesia is a major issue that can lead to abrasions, ulcerations, and visual loss. Facial dysesthesias, sometimes painful, can also occur.
In contrast to neuromodulatory approaches, bilateral destructive procedures are not recommended given the significant ophthalmologic risks. Studies supporting these procedures are often small, uncontrolled, and without long-term follow-up. Given the many new therapies and approaches, destructive procedures cannot be recommended.
• Migraine is highly prevalent and the most disabling of neurologic disorders.
• Migraine therapy is divided into acute treatment of attacks as they occur and preventive therapy which has the goal of reducing attack frequency.
• Regular, frequent use of acute medications (“medication overuse”) can increase migraine frequency; appropriate selection and limitation of abortive use is of utmost importance.
• Finding an effective migraine preventive may take time, as each medication should be trialed at an adequate dose for at least 2 months.
• Tension-type headache can often be managed with over-the-counter analgesics as needed. Tension-type headaches that are frequent enough to require preventive therapy can be treated with migraine preventives.
• Cluster headache is characterized by excruciating, unilateral attacks often associated with prominent, ipsilateral cranial autonomic symptoms. High-flow oxygen and injectable or inhaled triptans are the mainstay of acute therapy. Preventive therapy to suppress attacks may require a combination of short- and long-term strategies.
• Neuromodulation is an area of increasing interest and development for treatment of primary headache disorders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree