CHAPTER 319 Thoracolumbar Trauma
The incidence of thoracolumbar spine injuries after blunt trauma is approximately 2% to 7.5%.1–4 Nineteen percent to 50% of these injuries have associated neurological injury.5–7 Even in the absence of neurological deficits, injuries to the thoracolumbar spine can be associated with long-term pain and disability.8,9
Thoracolumbar fractures are the result of high-velocity deceleration mechanisms and occur most commonly in association with falls or motor vehicle accidents.1,10,11 The thoracolumbar spine is particularly vulnerable to injury in falls from a height greater than 3 m. Spinal and lower extremity fractures are the most common injuries after a fall from a height.12
Anatomy
The anatomy and biomechanical properties of the thoracolumbar spine dictate the patterns of injury that occur commonly. The transition between the more rigid thoracic spine and the mobile lumbar spine concentrates bending and axial loads at the thoracolumbar junction and thus explains the higher prevalence of injury at the T11-L1 level than at more proximal aspects of the thoracic spine or distal lumbar levels.13 Unfortunately, in adults the part of the spinal cord that ends near the mechanically vulnerable thoracolumbar junction contains the primary efferents for all the lumbosacral roots, and hence canal encroachment can have significant neurological consequences.
The anatomy of the rib cage significantly influences the biomechanical properties of the thoracolumbar spine. Above T10, ribs 3 through 8 articulate anteriorly with the sternum and posteriorly with their associated vertebral body and transverse process, as well as with the vertebral body above via an inferior demifacet. This configuration stabilizes the thoracic spine and increases its rigidity twofold to threefold.14 Below this level, the rigidity of the thoracic spine is reduced as the anatomy evolves into the configuration of the more mobile lumbar spine.
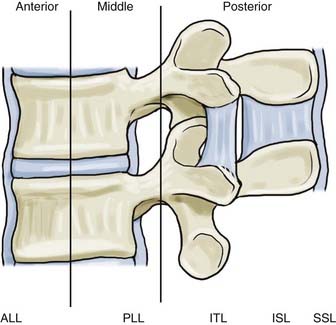
The recently proposed Thoracolumbar Injury Classification and Severity Score (TLICS) has emphasized the integrity of the posterior ligamentous complex (PLC) in determining stability and dictating treatment of thoracolumbar injuries.15 The PLC is composed of several individually named ligamentous structures, including (anterior to posterior) the facet joint capsules, ligamentum flavum (LF), interspinous ligament (ISL), and supraspinous ligament (SSL).
The LF in the thoracolumbar region has two layers, superficial and deep.16 The deep layer runs vertical and resists kyphotic deformation of the spine. The superficial layer contains a “zone of separation” between the right and left fibers, whereas the deep layer contains a prominent midline “cleavage separation.” Elastic fibers make up 80% of the LF and give it its characteristic yellow color. In the neutral position, the LF is actually in a stretched state. With aging, the elastic component is replaced with collagen and chondrocytes and at times may become ossified. The degenerative changes in the LF result in a decreased ability to resist flexion. The increased mass of the thickened LF can also contribute to neural compression in trauma.
The ISL becomes more developed as one moves caudally down the thoracolumbar spine. The ISL can be divided into anterior, middle, and posterior portions. The anterior portion is separated into a pair of ligaments with a thin sheet of adipose tissue between them. Each side is continuous with the corresponding LF anteriorly. The posterior portion is fused into a single ligament and blends with the SSL posteriorly. The middle portion makes up the majority of the ligament. The fibers actually run from anteroinferior to posterosuperior. This orientation does not limit flexion, but complex interactions between collagen fibers increase the ligament’s stiffness in flexion.17 In a cadaveric model, the ISL and SSL are the first to sprain during hyperflexion.18
The SSL, which runs along the entire length of the spine, becomes strongest in the lumbar spine. Some controversy exists whether it is a true ligament or whether it represents the strong tendinous fibers of the deep erector spinae and crossing fibers of the thoracolumbar fascia.19 Regardless, because of its orientation and distance from the axis of rotation, it is particularly effective in resisting flexion movements.
Thoracolumbar spinal fractures place the conus medullaris and cauda equina at risk, and injury to these neurological structures has profound functional consequences. The conus medullaris and cauda equina mark the transition between the central and peripheral nervous systems. Injury results in a constellation of upper and lower motoneuron symptoms, depending on the exact site of the lesion. The location of the conus medullaris varies by developmental stage. At birth, the cord fills the vertebral canal and terminates at the lumbosacral junction.20 The distal tip of the spinal cord then ascends with infant development, probably because of the differential growth between the spinal column and spinal cord. In adults, the tip usually terminates at the midaspect of the first lumbar vertebra. However, its position varies between the lower 11th thoracic and upper 3rd lumbar vertebrae.21 Traditionally, it has been taught that the termination also varies with the position of the spine, although a recent study by Bauer and colleagues found no change in the position of the conus with flexion.22 However, they did demonstrate that the cauda equina displaces medially over the conus with flexion.
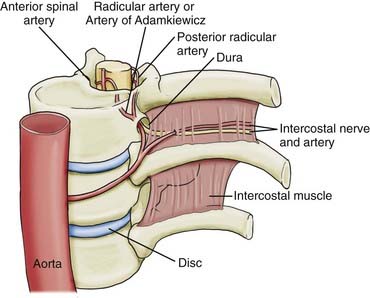
The origin of the artery is normally a direct or indirect branch of the left lower intercostal or upper lumbar arteries, between the 10th and 12th thoracic vertebrae (Fig. 319-1). Lu and coauthors reported in a cadaver study of 15 individuals that the artery was present on the left side as the primary radiculomedullary supply to the spinal cord in 80% of specimens.23 In the remaining 20% they found supplemental lumbar feeders lower down at L1 or L2 that anastomosed with the descending division. The vessel originated off the intercostal artery near the costotransverse joint before entering the intervertebral foramen on its intradural course. It is at risk for iatrogenic injury during approaches requiring disarticulation of the rib or sacrifice of the nerve root (e.g., in posterolateral approaches to the anterior thoracic spine).
Initial Assessment and Management
Clinical Evaluation
The secondary survey includes a complete neurological examination. This is best completed in the standardized format developed by the American Spinal Injury Association (ASIA).24 Certain myotomes are not tested with the standard ASIA form and should be noted specifically in patients with thoracolumbar trauma. The S2 myotome is documented by assessing the ability to flex the toes. Superficial cutaneous reflexes (abdominal cutaneous reflex and the cremasteric reflex) should also be documented.
As indicated earlier, at the thoracolumbar junction the spinal cord is ending and the cauda equina is emerging. Hence, injuries at this level can cause a constellation of neurological signs and symptoms as a result of varying degrees of spinal cord or peripheral nerve damage ![]() (Fig. 319-E1). Distinguishing the two in an individual with a T12 or L1 burst fracture can be extremely difficult, although conceptually, constituents of the peripheral nervous system have a greater chance of recovery after injury than do constituents of the central nervous system. Injuries isolated to the very tip of the conus medullaris where the sacral nerve roots emerge may cause profound deficits in bowel/bladder and sexual function with almost paradoxically normal lower extremity function. More typical, however, are mixed patterns of injury, given that the anatomy of the region makes it difficult to damage the distal aspect of the spinal cord without also damaging some of the roots of the cauda equina that are emerging at the same level. Fractures distal to L1 will affect the roots of the cauda equina more predominantly, depending obviously on how low the spinal cord extends in a given individual.
(Fig. 319-E1). Distinguishing the two in an individual with a T12 or L1 burst fracture can be extremely difficult, although conceptually, constituents of the peripheral nervous system have a greater chance of recovery after injury than do constituents of the central nervous system. Injuries isolated to the very tip of the conus medullaris where the sacral nerve roots emerge may cause profound deficits in bowel/bladder and sexual function with almost paradoxically normal lower extremity function. More typical, however, are mixed patterns of injury, given that the anatomy of the region makes it difficult to damage the distal aspect of the spinal cord without also damaging some of the roots of the cauda equina that are emerging at the same level. Fractures distal to L1 will affect the roots of the cauda equina more predominantly, depending obviously on how low the spinal cord extends in a given individual.
As in the cervical spine, the greatest danger in the assessment and early management of thoracolumbar injuries is missing the diagnosis. Delayed diagnosis occurs in almost 5% of thoracolumbar injuries, and such delays have been shown to increase the incidence of neurological deficits.8 Early recognition and prompt appropriate treatment are imperative to improve patient outcomes.
In a more recent study designed to develop imaging guidelines for the thoracolumbar spine in blunt trauma patients, Hsu and coworkers retrospectively applied a diagnostic pathway protocol based on a review of the literature.10 The majority of fractures in the study occurred at the thoracolumbar junction (62%) and were caused either by falls (49%) or by motor vehicle accidents (26%). The most common clinical sign of injury was the presence of back pain or midline tenderness (88%). Seven percent of patients had no detectable clinical signs. The clinical findings of back pain and midline tenderness are often difficult to ascertain in patients who are intoxicated, in those with a lowered Glasgow Coma Scale score, or in patients who have major distracting injuries.
Finally, a comment should be made regarding spinal immobilization and the use of spine boards. Spinal immobilization is used early during transportation to prevent neurological injury as a result of displacement of unstable injuries. Spine boards, in contrast, should act only as a transportation device and not as a means of providing ongoing immobilization once the patient has been evaluated. This distinction is sometimes lost on health care staff, who often view the spine board as a therapeutic measure for patients with thoracolumbar injuries, thereby resulting in prolonged periods of immobilization on a hard, flat surface. This has resulted in reported rates of pressure ulcers in trauma patients as high as 31%.25
Immobilization and spine boards are a known risk factor for pressure necrosis.26 In a study by Lerner and Moscati, the average time that a trauma patient spent on a spine board was approximately 1 hour.27 If patients required radiologic investigation before removal of the spine board, the total time reached 3 hours. Replacing the spine board with a supportive mattress is a simple maneuver that can prevent significant morbidity and mortality. It is important to remember that patients should be removed from the spine board as soon as possible after a qualified physician has assessed them.
Imaging
Imaging studies are ultimately required to make the diagnosis of thoracolumbar injuries and to guide management. To date, no standardized imaging algorithm for assessment of spine trauma has been established, although many institutions have developed their own protocols in an attempt to minimize the incidence of “missed injuries.” The most common cause of a missed thoracolumbar injury is inadequate imaging, which occurs not infrequently in the setting of a patient with urgent, life-threatening injuries.1,11
Despite variations in approach, some basic principles do exist for imaging patients with a thoracolumbar spine injury. Traditionally, imaging usually commenced with screening radiographs of the entire spine. More recently, however, the use of multidetector computed tomography (CT) in many emergency departments has begun to supplant conventional radiographic imaging. Plain radiographs continue to be useful, however, for identifying the level of injury, characterizing bony injuries (e.g., loss of height of the anterior vertebral body), assessing the relationship of one vertebral body to another, and evaluating the overall alignment of the spine.28
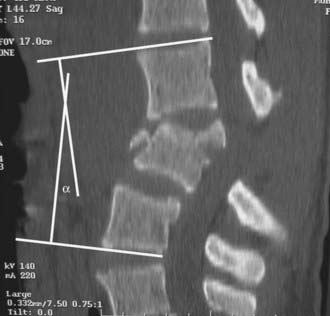
Review of the radiographic imaging requires a systematic approach to avoid overlooking injuries. Alignment should be assessed in the lateral and frontal planes by checking the anterior and posterior vertebral body lines, the spinolaminar line, the articular pillar and facet joints, the interpedicular and interspinous distances, and the position of the transverse processes. Translation of one vertebral body on the next should be evident in this assessment and probably reflects significant instability.29 Angulation across an injury site can be measured with the Cobb method, which involves measuring from the superior end plate one level above the fracture to the inferior end plate one level below the fracture (Fig. 319-2).30 On anteroposterior (AP) radiographs, widening of the interpedicular distance relative to other levels suggests failure of the posterior vertebral body (middle column), as seen in burst fractures. The interspinous distances can sometimes also be appreciated, and widening is suggestive of a flexion-distraction injury.
Radiographic signs that may indicate major ligamentous (including PLC) disruption and instability include displacement of bone, widening of the interlaminar space, widening of the apophyseal joints, widening of the vertebral canal, and disruption of the posterior vertebral body.31 Daffner and colleagues retrospectively reviewed 138 spinal injuries in 125 patients and noted an association of five radiographic findings with specific anatomic injuries (skeletal, ligamentous, and articular).32 They concluded that “the presence of only one is sufficient to establish a diagnosis of instability.” In an earlier cadaveric study, Nagel and associates were able to demonstrate, in the absence of a vertebral fracture, that kyphosis of 20 degrees or lateral angulation of 10 degrees or greater requires that the PLC and posterior anulus must be disrupted.33
Unfortunately, radiographs of the spine are often inadequate, especially in an obese patient. With advances in CT, plain radiography is taking on a more limited role. Multidetector CT allows excellent bone detail, and multiplanar reformations permit visualization in any plane. Three-dimensional constructions add a spatial appreciation of the fracture pattern. CT also adds the benefit of visceral imaging. Abdominal and pelvic injury is common in patients with thoracolumbar injuries.34
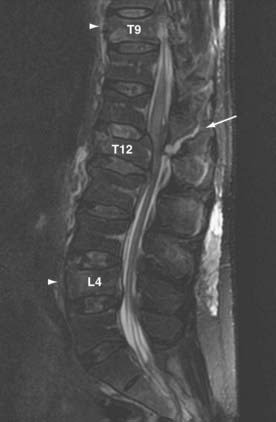
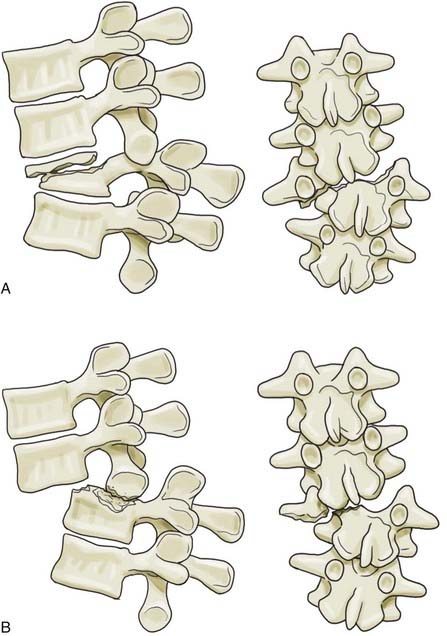
Once a spine injury is identified, it is prudent to obtain a CT scan or further radiographs of the entire spine to rule out other noncontiguous fractures. Approximately 4.2% to 23.8% of patients have noncontiguous fractures on plain radiographic studies.35 These most commonly occur at the cervicothoracic and lumbosacral junctions. In a prospective study of all spine trauma patients admitted over a 3-year period, Green and Saifuddin documented a 34% incidence of noncontiguous injuries with magnetic resonance imaging (MRI) (21% for noncontiguous wedge compression fractures and 13% for noncontiguous burst fractures)36 (Fig. 319-3).
MRI in patients with thoracolumbar trauma is complementary to CT scanning. The obvious benefit is the ability to visualize the diskoligamentous soft tissues and neurological structures. The anatomy of the neural elements (spinal cord, conus medullaris, cauda equina, and nerve roots) is important to visualize in patients with neurological deficits. Epidural hematomas and their relationship to the spinal cord can be appreciated. The great medullary artery can be detected in the majority of patients with contrast-enhanced magnetic resonance angiography.37
Because the diskoligamentous structures (and particularly the PLC, which consists of the SSL, ISL, LF, and facet joint capsules) play such an important role in determining the stability of the injury, assessment of these soft tissue structures with MRI can be extremely helpful in guiding management decisions. In fact, there is a general consensus among leading spine surgeons that disruption of the PLC makes a thoracolumbar spinal injury much more unstable and warrants strong consideration for operative intervention.29
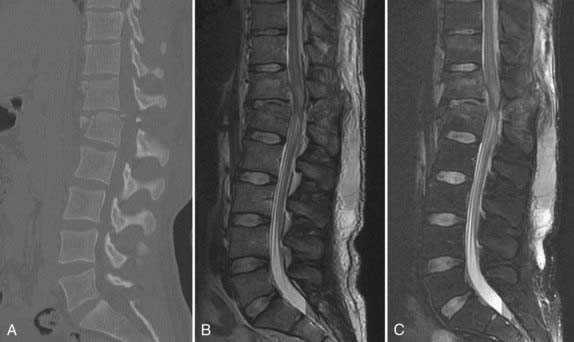
Although plain radiography and CT rely on relative displacement or angulation of adjacent vertebral segments as indirect signs of PLC disruption, MRI may be able to directly detect disruption of the PLC.35,38,39 The sensitivity and specificity of MRI in predicting a torn PLC are 90% and 100%, respectively.39 What defines PCL disruption and which imaging sequence is used to do so vary between studies. Discontinuity of the “black stripe” on T2-weighted MRI, which represents the LF, posterior longitudinal ligament, or SSL, is often used. Tears of the facet capsule and the ISL result in accumulation of fluid and is represented by high-intensity signal on T2-weighted imaging. The high-intensity changes related to acute edema can be difficult to distinguish from fat, which has signal intensity similar to that of edema on standard T2-weighted imaging. T2-weighted fat suppression protocols remove the surrounding “noise” caused by fat and improve the ability to discern acute injuries (Fig. 319-4). In an important study, Lee and associates documented the clinical, radiographic, and MRI findings in patients with thoracolumbar injuries to assess their reliability in accurately predicting injury to the PLC, which was subsequently evaluated intraoperatively40 ![]() (Table 319-E1). The accuracy of palpation, plain radiography, and MRI for correctly predicting disruption of the PLC (SSL/ISL/LF) was 53.6%, 66.7%, and 90.9%/97%/87.9%, respectively. It should be noted that palpation and plain radiography had excellent positive predictive values (92.9% and 95.2%, respectively), which suggests that these tests are actually quite good in identifying injuries but that MRI may have a stronger role when clinical examination or plain radiology is negative or indeterminate. Unfortunately, standardized criteria for defining PLC integrity do not currently exist, and accordingly, even experienced surgeons demonstrate only fair agreement when assessing MRI studies to determine the integrity of the PLC.15,29 Further refinements in MRI protocols and criteria for establishing the integrity of the PLC are needed.
(Table 319-E1). The accuracy of palpation, plain radiography, and MRI for correctly predicting disruption of the PLC (SSL/ISL/LF) was 53.6%, 66.7%, and 90.9%/97%/87.9%, respectively. It should be noted that palpation and plain radiography had excellent positive predictive values (92.9% and 95.2%, respectively), which suggests that these tests are actually quite good in identifying injuries but that MRI may have a stronger role when clinical examination or plain radiology is negative or indeterminate. Unfortunately, standardized criteria for defining PLC integrity do not currently exist, and accordingly, even experienced surgeons demonstrate only fair agreement when assessing MRI studies to determine the integrity of the PLC.15,29 Further refinements in MRI protocols and criteria for establishing the integrity of the PLC are needed.
Despite the increased use of MRI in patients with thoracolumbar trauma, many spine surgeons believe that plain AP and lateral radiographs are the most important factors in defining PLC integrity.41 This is consistent with Daffner and colleagues’ work on radiographic signs.32 Members of the Spine Trauma Study Group (STSG) were surveyed to identify imaging parameters that they thought were most suggestive of PLC disruption in the thoracolumbar spine. “Vertebral body translation” on plain radiographs was ranked the most important indicator of PLC disruption by 50% of respondents, with “interspinous spacing greater than the level above or below on AP plain X-ray” and “diastasis of the facet joints on CT” being ranked second and third, respectively. Surprisingly, MRI indicators of PLC disruption ranked lower. In essence, this simply reflects the fact that when gross malalignment of the spine exists, there is little doubt about the integrity of the PLC.
Classification
Fracture classification systems attempt to produce a universal nomenclature to facilitate communication between health care members, guide patient management, and allow consistent stratification for ongoing research. Classification categories should be clinically relevant entities that surgeons use for diagnosis with sufficient confidence to limit incorrect categorization and associated treatment errors. No classification system of thoracolumbar injuries has been universally accepted to date. Historically, thoracolumbar spine classification systems have been scrutinized for their reliability, reproducibility, or clinical validity.42–44 As a result, classification systems for thoracolumbar trauma continue to evolve.
The first generation of thoracolumbar fracture classification systems focused on describing common fracture patterns. Bohler in 1930 published the first thoracolumbar classification system.45 He used fracture pattern and mechanism of injury to distinguish five fracture subtypes: compression, flexion-distraction, extension, shear, and rotational injuries. He did not attempt to classify injuries as stable or unstable based on fracture pattern. In 1943, Watson-Jones published a book in which he described the concept of instability in spinal fractures.15a Nicholl expanded on this work by identifying the instrumental role of the PLC.16
The second generation of thoracolumbar fracture classification systems used architectonic abstractions, most notably the concept of spinal “columns,” in an attempt to predict the relationship of different fracture patterns to immediate and long-term mechanical and neurological instability. Holdsworth developed the two-column concept in which the vertebral body acted as the anterior weight-bearing column, with the stabilizing ligamentous complex being located posteriorly.46 Disruption of the PLC defined instability. Biomechanical studies, however, demonstrated that disruption of the PLC in conjunction with the vertebral body (middle and anterior columns) was required to produce instability.16,47 Holdsworth’s two-column concept did not allow for this.
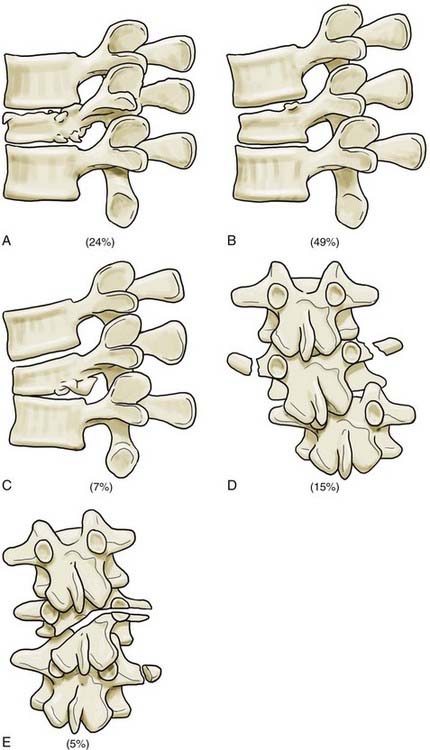
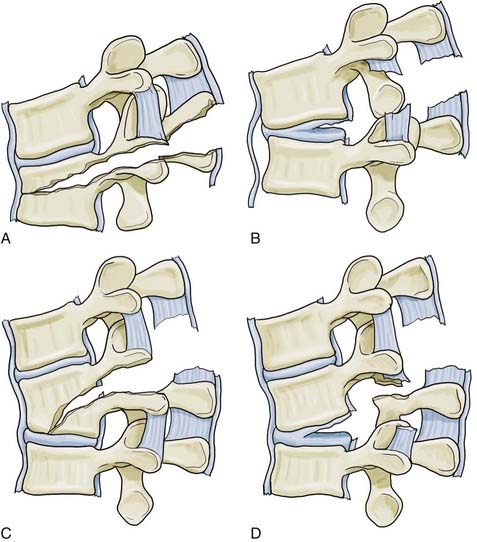
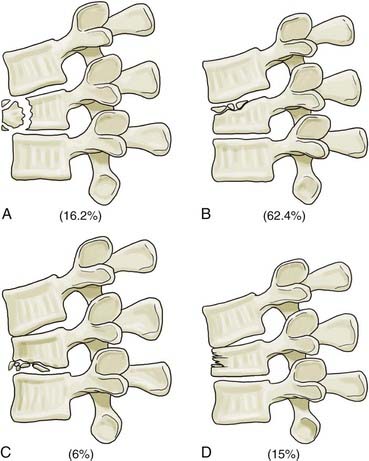
The third generation of thoracolumbar fracture classification systems is marked by the advent of CT, which dramatically increased the ability to describe fracture morphology in detail. Based on this, Denis48 and McAfee and colleagues49 simultaneously introduced the three-column concept of the spine in 1983. Denis’ retrospective review of 412 thoracolumbar injuries added the middle column to Holdsworth’s two-column model. The anterior column included the anterior longitudinal ligament and the anterior half of the disk and vertebral body. The middle column included the posterior longitudinal ligament and the posterior half of the disk and body. The posterior column included the remaining elements (Fig. 319-5). According to Denis, failure of the middle column was key in defining “instability.” Four fracture patterns were defined on the basis of relative involvement of each column: compression, burst, seat belttype, and fracture-dislocation. These four types were then divided into groups and subgroups, which resulted in 16 total fracture subtypes (Figs. 319-6 to 319-8 and Fig. 319-E2). ![]() Burst fractures, based on disruption of the middle column, were classified as being mechanically unstable, but this concept has subsequently generated substantial confusion given that we now recognize that such injuries are usually stable if the PLC is intact.
Burst fractures, based on disruption of the middle column, were classified as being mechanically unstable, but this concept has subsequently generated substantial confusion given that we now recognize that such injuries are usually stable if the PLC is intact.
The Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification was introduced by Magerl and colleagues in 1994.50 It is the most widely used system in European centers. The AO classification is based on mechanistic and morphologic criteria. Within each mechanistic class, further subclassification reflects a graduated scale of progressive injury and instability (Table 319-E2). ![]() The classification system is built on a 3-3-3 scheme. The fundamental injury pattern is determined by the mechanism of injury: A, compression; B, distraction; and C, axial torque. Each mechanistic type is further subdivided into three groups based on morphologic severity and then into three subgroups. As result of its complexity, the reliability of the system is compromised.44,51 Wood and associates evaluated the reliability of the AO and Denis classification systems. Thirty-one acute thoracolumbar spine fractures were classified with each system on two occasions 3 months apart by 19 trained spine surgeons. The mean interrater reliability was only moderate for both systems (AO classification, κ = 0.48; Denis classification, κ = 0.54). Intrarater reliability was substantial for the basic level of classification (A, B, and C in the AO classification; compression, burst, seat belt, and fracture-dislocation in the Denis classification). With further subclassification, the reliability was significantly diminished (no κ value given). The authors pointed out the concern over the “tendency for well trained spine surgeons to classify the same fracture differently.”
The classification system is built on a 3-3-3 scheme. The fundamental injury pattern is determined by the mechanism of injury: A, compression; B, distraction; and C, axial torque. Each mechanistic type is further subdivided into three groups based on morphologic severity and then into three subgroups. As result of its complexity, the reliability of the system is compromised.44,51 Wood and associates evaluated the reliability of the AO and Denis classification systems. Thirty-one acute thoracolumbar spine fractures were classified with each system on two occasions 3 months apart by 19 trained spine surgeons. The mean interrater reliability was only moderate for both systems (AO classification, κ = 0.48; Denis classification, κ = 0.54). Intrarater reliability was substantial for the basic level of classification (A, B, and C in the AO classification; compression, burst, seat belt, and fracture-dislocation in the Denis classification). With further subclassification, the reliability was significantly diminished (no κ value given). The authors pointed out the concern over the “tendency for well trained spine surgeons to classify the same fracture differently.”
TABLE 319-E2 AO Classification
| Type A: Vertebral Body Compression | ||
| A1. Impaction fractures | A1.1. End-plate fractures | |
| A1.2. Wedge impaction fractures | ||
| A1.3. Vertebral body collapse | ||
| A2. Split fractures | A2.1. Sagittal split fracture | |
| A2.2. Coronal split fracture | ||
| A2.3. Pincer fracture | ||
| A3. Burst fractures | A3.1. Incomplete burst fractures | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|