CHAPTER 306 Thoracoscopic Approaches to the Spine
Endoscopic techniques in spinal surgery are now common for a variety of pathologies: posterolateral percutaneous approaches to the lumbar disk spaces and neural foramina, anterior laparoscopic and anterolateral retroperitoneal endoscopic approaches to the lumbar spine, and thoracoscopic approaches to the thoracic spine.1–14 Typically, rigid rod-lens endoscopes are used to visualize the anatomy and pathology; however, flexible fiberoptic endoscopes have also been used to inspect small spaces such as the neural foramina and syringomyelia cavities.7–10,13,15 The resolution and image quality of flexible fiberoptic endoscopes are poorer than that of rigid endoscopes.
Endoscopes have found a valuable place in the treatment of thoracic spinal disorders. Thoracoscopy was first widely employed by cardiothoracic surgeons, and the techniques for thoracoscopic spinal surgery are adapted from their methodologies.16–19 Today, thoracoscopic surgical techniques are used to perform sympathectomies, discectomies, and vertebrectomies; to correct deformities; to stabilize spine fractures after trauma; and to biopsy and resect tumors.
Historical Overview
Beginning in the early 1900s, thoracoscopy was used as a diagnostic tool to evaluate pleural disease.20–23 During the late 1980s, techniques and instrumentation for endoscopic surgical procedures improved dramatically. In the early 1990s, thoracoscopic techniques were refined and applied to a broad spectrum of pathologies involving the thorax.16–19
Today, many thoracic procedures previously performed via a thoracotomy are routinely performed thoracoscopically. These procedures include biopsy or resection of pleural or lung lesions, lymph node biopsy, biopsy and resection of mediastinal masses, lobectomy, pneumonectomy, pleural sclerotherapy, treatment of blebs, esophageal procedures, and sympathectomy.16–1924 In the resection of pulmonary lesions,24–26 the small thoracoscopic incisions have minimized dissection and retraction of the chest wall, reduced postoperative pain, decreased blood loss, shortened intensive care unit and overall hospital stays, improved postoperative pulmonary and shoulder function, hastened recovery times, and decreased complications.16–19,24–26
The techniques of thoracoscopic spine surgery were independently developed by Regan and coworkers3,6 in the United States and by Rosenthal and colleagues5,27 in Germany. The first report of thoracoscopy for spinal diseases was published by Mack and coworkers28 who described 10 patients with diverse spinal pathology effectively treated thoracoscopically without major complications. Rosenthal and associates5 and Horowitz and coworkers4 published separate reports that described the techniques for performing thoracic microdiscectomy thoracoscopically. Since then, numerous reports have demonstrated the effectiveness of thoracoscopic spinal surgery for the treatment of a wide variety of spinal disorders.16,29–31
Indications
Thoracoscopy can be used to access the sympathetic chain, disks, vertebral bodies, and the ipsilateral pedicle; however, it cannot be used to access the posterior elements of the spine. Thoracoscopic approaches have been used to treat herniated thoracic disks2–628; to drain vertebral epidural abscesses; to débride vertebral osteomyelitis and diskitis; to decompress fractures; to biopsy and resect neoplasms1–328; and to perform vertebrectomies and interbody fusions, vertebral body reconstructions and instrumentation,1–3,28,30 sympathectomies,32–34 and anterior releases for the treatment of kyphosis and scoliosis (Table 306-1).2,3,6,28,30
Table 306-1 Potential Indications for Thoracoscopic Spinal Surgery
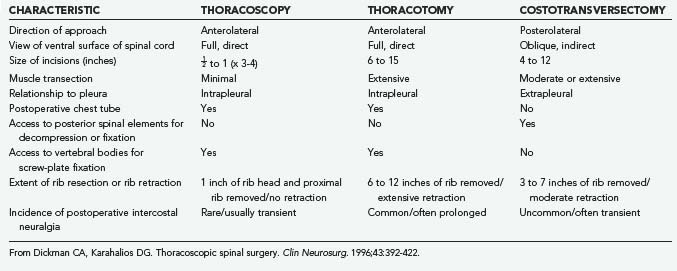
Costotransversectomy, thoracotomy, and thoracoscopy are the three major techniques available to address thoracic vertebral and disk pathologies. Each technique has distinct advantages and disadvantages (Table 306-2). When the ventral aspect of the dura must be visualized well, an anterior transthoracic approach (thoracotomy or thoracoscopy) is necessary. This significantly improves visualization of the ventral surfaces of the spine and spinal cord to facilitate decompression, reconstruction, and internal fixation compared with posterolateral approaches.35–44 For lateral pathologies, a costotransversectomy, transpedicular approach, or other such posterolateral approaches may be considered.
Contraindications
Contraindications to a thoracoscopic approach are similar to those for open approaches and include medical reasons that would prohibit surgery (uncontrollable coagulopathy, terminal illness, or severe cardiac or pulmonary disease). Requirements specific to thoracoscopic approaches include the ability to tolerate prolonged single-lung ventilation and the absence of significant pleural adhesions or advanced pulmonary disease. Patients with conditions such as chest trauma, a prior thoracotomy, emphysema, or hemothorax may have extensive adhesions that prohibit thoracoscopic access. Extensive scar tissue from an earlier operation at the site of spinal pathology also precludes thoracoscopy. Consequently, most patients should be evaluated before surgery by a pulmonologist or internist, as well as by a cardiothoracic surgeon when indicated. The preoperative assessment can include spirometry, blood gases, and pulmonary function studies as needed (Table 306-3).
Table 306-3 Contraindications for Thoracoscopic Procedures
Educational Issues
Thoracoscopy represents a technique that is fundamentally unfamiliar to most spinal surgeons. Because of the restricted portals of entry, thoracoscopic techniques require new psychomotor skills for navigating and manipulating instruments from a distance while watching the procedure in real time on a video monitor. The clinical application of thoracoscopic techniques should only follow a comprehensive training program that includes didactic and practical components. Extensive practice in a surgical skills laboratory in either animal or human models is mandatory. Procedures also should be performed with the assistance of a cardiothoracic surgeon so that open exposure can be performed immediately if needed. Although there are no specific guidelines for the practice of thoracoscopic spinal surgery, the cardiothoracic surgery community has outlined the ethical and educational issues relating to the use of this technique by their practitioners.15,45
Thoracoscopic Technique
Operating Room Setup and Patient Positioning
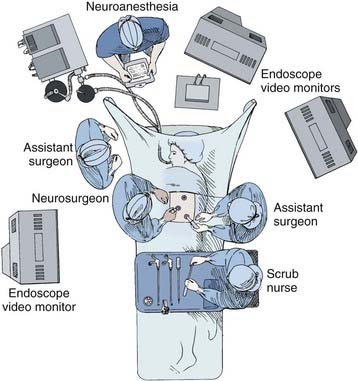
A radiolucent operating table is used so that fluoroscopic images can be obtained intraoperatively. Initially, the patient is placed supine on the operating room table while a double-lumen endotracheal tube is placed. Fiberoptic bronchoscopic equipment should be kept in the room should the endotracheal tube need to be repositioned or the patient suctioned during the procedure. Typically, the anesthesiologist is positioned at the head of the operating table (Fig. 306-1). An arterial line, central venous and urinary catheters, and pneumatic compression stockings are placed. Somatosensory and/or motor evoked potential leads are connected and baseline recordings are obtained before the patient is positioned.
Portal Insertion
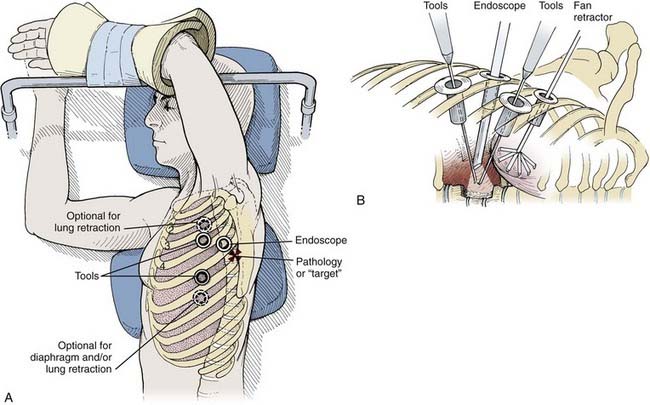
Depending on the procedure, two to four portals are inserted to gain access to the thoracic cavity (Fig. 306-2). The portals should be spread far enough apart so that the surgeon’s hands are neither too close together nor too close to the endoscope. The working portals (for instruments) are best positioned anterolaterally between the anterior and middle axillary lines. The endoscope portal is best positioned posterolaterally between the middle and posterior axillary line. This technique allows the surgeon’s hands to rest comfortably during the procedure. The axilla and first and second interspaces are never entered to avoid injury to the brachial plexus and great vessels, respectively. Exposure from T9 to T12 requires caudal retraction of the diaphragm to expose the costophrenic recess. This exposure can be enhanced by a reverse Trendelenburg position and a fan retractor.
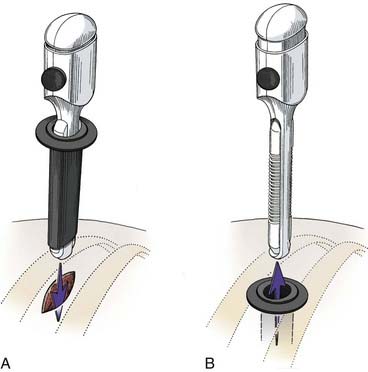
Before the portals are placed, the skin is infiltrated and an intercostal nerve block is administered with a local anesthetic (1% bupivicaine [Marcaine] with epinephrine). The skin is incised parallel to the superior surface of the rib to prevent injury to the neurovascular bundle. A hemostat is passed through the intercostal muscles and parietal pleura directly adjacent to the superior surface of the rib. A finger can be inserted to check for lung adhesions that would preclude the introduction of a portal at that site. Portals are placed over a rigid trocar, which is immediately removed after the portals have been placed (Fig. 306-3). The proximal end of the portal is stapled or sutured to the skin to anchor it to the chest wall during surgery.
Thoracic Endoscopic Sympathectomy
Several clinical syndromes that result from a pathologically elevated sympathetic tone can be treated surgically by thoracic sympathectomy. These entities include palmar or axillary hyperhidrosis, pain syndromes involving the upper extremities such as reflex sympathetic dystrophy (RSD), ischemic syndromes of the hand such as Raynaud’s disease, and malignant tachyarrhythmias refractory to medical management. The second, third, and sometimes fourth sympathetic ganglia are thought to be the primary mediators of these disease processes. Traditionally, the second thoracic ganglion is considered to be the key ganglion for sympathetic denervation of the upper extremity.2 Thoracic endoscopic sympathectomy, a technique first described about 50 years ago,3 provides an appealing alternative for patients with conditions that are treatable by sympathetic denervation.
Surgical Indications
Several major groups of disorders can be treated by thoracoscopic sympathectomy (Table 306-4) and contraindications for the procedure are few. Idiopathic (essential) palmar hyperhidrosis is the most common indication for thoracoscopic sympathectomy. Most patients who receive a neurosurgical referral for this condition have been evaluated for metabolic (hyperthyroidism) or neoplastic causes and have failed efforts at medical management with topical and anticholinergic agents.
Table 306-4 Indications for Sympathectomy
From Dickman CA, Baskin JJ, Theodore N. Thoracic endoscopic sympathectomy. In: Fessler RG, Sekhar LN, eds. Atlas of Neurosurgical Techniques. New York: Thieme; 2006.
In clinical series, the success rate of sympathectomy for permanent relief of palmar hyperhidrosis ranges from 90% to 100%.33,34,45–47,51,54–58,62,64 Axillary hyperhidrosis and bromhidrosis (axillary malodor) can also be addressed through a sympathectomy that targets the T3 and T4 ganglia.5 Associated plantar hyperhidrosis often (50%) resolves when hyperhidrosis of the upper extremities is relieved and is referred to as a dividend benefit of the procedure because it is not an expected effect of transecting the upper thoracic sympathetic chain.
RSD (also known as complex regional pain syndrome type I)56 is one of a number of pain syndromes that typically follow trauma. Current evidence suggests that an upregulated sensitivity of α adrenoreceptors for catecholamines in the injured limb reduces RSD. Medical therapy tends to be ineffective in terms of both the degree and duration of relief. Patients who experience symptomatic relief after percutaneous blocks of the stellate ganglion with local anesthetic agents are considered candidates for surgical sympathectomy.33,49,54–56 Long-term clinical benefits have been reported for this indication in more than 50% of patients.2
Patients with severe upper extremity ischemia due to Raynaud’s disease or related disorders may also benefit from sympathectomy.33,49,54–56 Although the ischemic process is typically progressive, sympathectomy can be used to avoid limb amputation and to improve the associated complaints of pains.
Sympathectomy has also been shown to effectively relieve pain related to pancreatic carcinoma via left T5 through T11 lesioning,59 as well as treating patients with increased QT intervals via T1 through T4 lesioning.60,61
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree