Chapter 121 Thoracoscopic Sympathectomy for Hyperhidrosis
Axillary and palmar hyperhidrosis are relatively common disorders that affect approximately 0.5% to 1% of the population, with possibly higher percentages in those of Asian descent.1 Hyperhidrosis is an idiopathic overactivation of sweat glands that results in secretion of sweat in excess of that needed for typical autonomically controlled thermoregulation. It is often overlooked or untreated and can cause significant distress that may lead to a negative impact on social and professional quality of life.2 Simple tasks such as handshaking, writing, and handling daily objects can become daunting problems. It can become so severe that sweat can drip off a person’s fingertips and can even require multiple changes of clothing daily. Patients with untreated hyperhidrosis have an increased risk for cutaneous skin infection if the abnormal physiology is not adequately corrected. Walling determined the overall odds ratio for infection of untreated hyperhidrosis is 3.2, for dermatophyte fungus is 9.8, and for bacteria is 2.6.3
Diagnosis criteria stratify severity into four classes, according to the Hyperhidrosis Disease Severity Scale: never noticeable, tolerable but interferes, barely tolerable and frequently interferes, and intolerable and always interferes.4 Additional criteria include duration in the previous 6 months; bilateral with symmetry; onset before 25 years of age; family history; and asymptomatic during sleep.5
History
While the earliest surveys of the sympathetic nervous system were conducted by du Petit in 1727, the first report of surgical sympathectomy was at the level of the neck for treatment of epilepsy by Alexander in 1889.6,7 Since then, it has been used for numerous clinical presentations with limited success. For example, sympathectomy has been indicated in treatment of angina,8 Raynaud phenomenon,9 exophthalmic goiter,10 glaucoma,11 various pain conditions,12 and spastic paralysis of lower extremities.13
Bernard and Horner independently published a series of reports in the latter half of the 19th century describing sectioning of the sympathetic chain and associated clinical effects.13a13b Since the 1920s, when Kotzareff described anhidrosis with sectioning of the sympathetic chain,14 sympathectomy has been commonly used for hyperhidrosis. The adaptation of endoscopic surgery was introduced in the 1940s when Hughes described an endoscopic approach for thoracic sympathectomy.15
Anatomy and Physiology
The sweat response is under hypothalamic thermoregulatory control via the preoptic sweat area.16 Autonomic output to eccrine glands arise both from input responding to thermoregulatory and from emotional state. Therefore, heightened emotions trigger a sweat response, such as sweaty palms with anxiety or nervousness.
Importantly, approximately 10% of the population carry the nerve of Kuntz, an additional aberrant division of the sympathetic chain arising from T1, T2, or T3.17 If present, the nerve of Kuntz must be sectioned to assure that sympathectomy will be effective.
Operative Technique
Patient Selection
Careful patient selection is critical for success. Prior to consideration for surgery, patients should have completed and failed a range of appropriate medical therapies. The mainstay of medical therapy includes over-the-counter (OTC) antiperspirants and topical 20% AlCl compounds. Chronic use of such antiperspirants can be limited by skin irritation, however. Medications that block alpha-adrenergic receptors such as phenoxybenzamine may also help symptoms. Often, these medications are poorly tolerated, as frequent side effects include hypotension and sexual dysfunction. Another more invasive option consists of a series of topical injections of botulinum toxin A (Botox). For palmar hyperhidrosis, an average of 26 Botox injections were required for determination of success or failure.18 Typically, even successfully treated patients need repeated injections after several months. Also, iontophoresis treatment can be tried prior to consideration of surgical treatment. Iontophoresis includes electrical stimulation, often with tap water, and can be conducted at home. However, it requires a significant daily time commitment. Lastly, in selected patients, imaging with computed tomography or magnetic resonance can be useful in excluding alternative pathologies, including infection, metabolic disorders, and neoplastic processes. If hyperhidrosis remains debilitating despite some of these interventions, surgery should be considered.
Anesthesia
Endoscopic thoracic sympathectomy typically requires placement of a double-lumen endotracheal tube. This allows for deflation of the lung on the operative side while allowing for ventilation of the contralateral lung. Alternatively, single-lumen endotracheal tube may be used with positive pressure carbon dioxide insufflation.19
Instruments
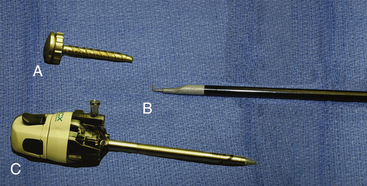
We typically use a 5-mm rigid endoscope for thoracoscopic procedures. Lens angle depends upon surgeon preference. We typically prefer a 30-degree angled scope. During the procedure, a dissection tool such as a cautery hook (Fig. 121-1) or scissors is necessary, as well as cotton pledgets and a suction irrigator.
Positioning
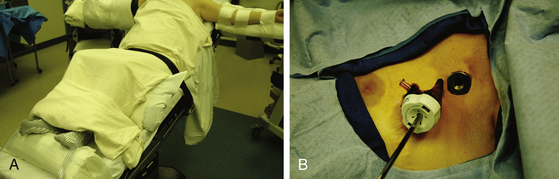
The patient should be positioned supine with the arms on arm boards perpendicular to the bed (Fig. 121-2). A reverse Trendelenburg position to approximately 40 degrees enables the deflated lung to fall away by gravity and minimizes the need for retraction. A footboard attached to the surgical table may be necessary to prevent the patient from sliding along the inclined bed.
Procedure
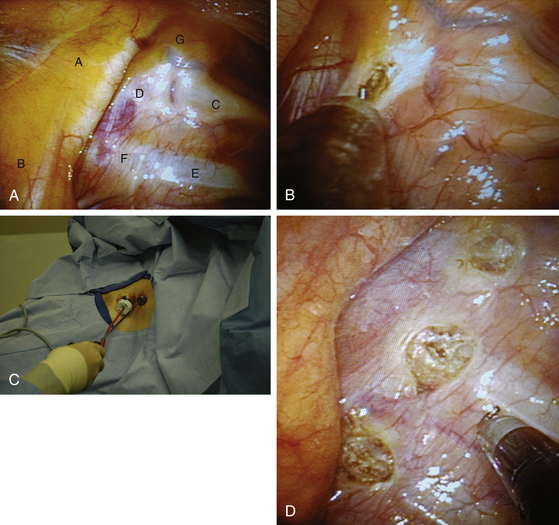
In the multiport method, two 5-mm thoracoscopic ports are placed in the third and fourth intercostal spaces (Fig. 121-2). The endoscope is introduced through one, and the second serves as a working port for sectioning the sympathetic chain. Alternatively, the procedure may be performed through a single port.20
The sympathetic chain is identified using the rib heads as landmarks. The first rib is uniquely curved like a sickle (Fig. 121-3A). It is associated with a prominent fat pad containing the first sympathetic ganglion, otherwise known as the stellate ganglion. The white sympathetic chain is seen coursing over the rib heads with the swelling of the ganglia close to the respective rib. Following adequate identification, the T2 to T4 portions of the sympathetic chain can be disconnected or clipped or excised.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree