Chapter 50B Trauma of the Nervous System
Craniocerebral Trauma
Classification
Traumatic brain injury is classified by mechanism, severity, and morphology (Table 50B.1).
Mechanism
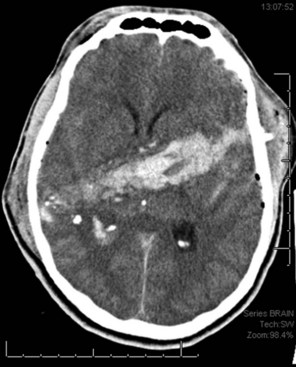
Gunshot wounds are the most prevalent penetrating head injuries (Fig. 50B.1), accounting for 35% of U.S. deaths from TBI in individuals younger than age 45. Self-inflicted injuries—for example, with power tools like nail guns (Fig. 50B.2)—are another cause of craniocerebral trauma (Testerman and Dacks, 2007), but gunshot wounds are the most lethal type of TBI, 90% of them resulting in death. They cause soft-tissue damage, often comminuted depressed skull fractures, and direct injury to the brain tissue from the missile. Beyond the laceration along the bullet path, further damage is done by shock waves, especially with high-velocity weapons. Depending on the type of penetrating injury, contamination may be a concern. However, bullet fragments are considered sterile owing to their exposure to heat from the firearm and are not routinely removed.
Vascular injury can occur with any type of TBI but is more common with penetrating trauma (25%–36% incidence) than with blunt injuries (<1%). Traumatic aneurysms, or pseudoaneurysms, can develop when all layers of the vessel wall rupture and surrounding cerebral tissue forms the aneurysmal wall. Such pseudoaneurysms can present as delayed subarachnoid hemorrhage (SAH) and can lead to death or severe disability. Therefore patients with penetrating injuries routinely undergo a cerebral angiogram to screen for traumatic pseudoaneurysm. A follow-up angiogram within the first months after a penetrating injury is recommended (du Trevou and van Dellen, 1992).
Blast is a rare mechanism of injury in civilian life, but it is common in combat. With ongoing militarily conflicts, blast injury has become more frequent in U.S. military personnel. American military members who returned from deployment to Iraq or Afghanistan self-reported a 12% to 20% incidence of mild TBI; explosions were recognized as a common injury mechanism (Wolf et al., 2009). Blast injuries can occur (1) as a direct result of supersonic waves of intense air (primary blast injury), (2) from being struck by objects set in motion by the blast (secondary blast injury), and (3) when a person is forcefully propelled by the blast (tertiary blast injury) (DePalma et al., 2005; Kocsis and Tessler, 2009). The brain is obviously vulnerable to secondary and tertiary blast injury, but whether primary blast forces directly injure the brain is controversial (Taber et al., 2006). The severity of blast exposure necessary to cause persistent symptoms is unclear (Hicks et al., 2010).
The severity of injury resulting from exposure to a blast can range from mild to fatal. Displacement, stretching, and shearing forces of the primary blast wave can affect the brain directly and lead to such sequelae as concussion, hemorrhage, severe edema, or diffuse axonal injury (DAI). Systemic acute air embolism from pulmonary disruption is believed to occlude the blood vessels of the brain or spinal cord and lead to stroke (Guy et al., 2000; Wolf et al., 2009). Reports from war zones suggest that brain swelling occurs within hours after a blast injury, and that the risk of mortality can be decreased by early decompressive craniectomy. Others have found that cerebral vasospasm occurred more often after a blast injury than with blunt TBI and led to a worse outcome than blunt severe TBI. Blast exposure on the mild end of the spectrum led to somatic, behavioral, psychological, and cognitive symptoms similar to postconcussion syndrome. There is well-recognized overlap between posttraumatic stress disorder and blast injury, and misdiagnosis can occur in both directions.
Injury Severity
There are different methods to stratify TBI by severity, and all are arbitrary. The most commonly used is the Glasgow Coma Scale (GCS), which was first published in 1974 by Graham Teasdale and Bryan K. Jennett, two neurosurgeons at the University of Glasgow (Teasdale and Jennett, 1974). The GCS assesses level of consciousness. It consist of three subscores: eye opening (maximal score: 4), verbal response (maximal score: 5), and motor response (maximal score: 6). Despite some shortcomings, it has excellent interrater agreement (weighted kappa >0.75) for verbal and total scores and intermediate agreement (weighted kappa 0.4-0.75) for motor and eye scores (Holdgate et al., 2006). Today it has been widely adopted by emergency medicine services and physicians to communicate the severity of injury in a consistent way. However, it is not designed to detect focal neurological deficits and is not a replacement for a thorough neurological examination.
A GCS score of 15 to 13 is considered to indicate mild TBI, and a score of 8 or below is a universally accepted definition of coma and severe head injury. Scores between 13 and 8 are classified as moderate. It is true that moderate and severe TBI are rare; mild TBI is eight times more common. Moderate and severe TBI are seen equally often. One can imagine that the GCS score shows significant ceiling effect in patients with mild TBI. Therefore, more sensitive measures exist for this purpose. The two most often used are the Cantu system (Cantu, 1996) and the American Academy of Neurology system (1997).
Despite its high incidence, no objective test to diagnose mild TBI currently exists. Only one of ten head CT scans has a positive finding in mild TBI patients. Therefore, special factors need to be considered when determining the need of a CT scan (Box 50B.1). Diagnosis is hampered by the lack of obvious injuries on computed tomography (CT) or conventional magnetic resonance imaging (MRI) and usually is based solely on clinical symptoms. Preliminary studies are investigating brain injury biomarkers to diagnose mild TBI (Dash et al., 2010; Hergenroeder et al., 2008; Kochanek et al., 2008). Although the initial progress has been promising, these markers are not yet used in the clinical setting (Springborg et al., 2009; Unden and Romner, 2009). Others have shown that an integrated approach with magnetoencephalography and diffusion tensor imaging (DTI) is more sensitive than conventional CT and MRI in detecting subtle neuronal injury in mild TBI and postconcussion syndrome (Huang et al., 2009).
Box 50B.1 Factors to Consider When Determining Need of CT in Patients with Head Injury
CT, Computed tomography; GCS, Glasgow Coma Scale; LOC, loss of consciousness.
A concussion is defined as injury to the brain caused by a hard blow or violent shaking, producing a sudden and temporary impairment of brain function such as a brief loss of consciousness or disturbance of vision and equilibrium. Concussions are equivalent to a mild TBI with negative CT findings. Their annual incidence in the United States is 128 per 100,000 population (Ropper and Gorson, 2007). The loss of consciousness is believed to result from rotational forces exerted on the upper midbrain and thalamus, impairing the function of the reticular neurons. Headache, nausea, dizziness, irritability, and impaired ability to concentrate can persist for days after the event. Persistence of these symptoms for weeks is called postconcussion syndrome and can last from 1 month to a year.
Patients with severe TBI (GCS score ≤ 8) often have injuries in at least one other organ system (Saul and Ducker, 1982). There is also a 5% incidence of associated spine fractures. About one-fourth of patients with a severe TBI undergo neurosurgical intervention. Early diagnosis of TBI improves outcome by reducing secondary injury, which can develop subsequent to the impact and includes edema, hypoxia, hypotension, ischemia, and elevated intracranial pressure (ICP) (Chesnut et al., 1993a; Chesnut et al., 1993b).
Morphology
Traumatic brain injury can also be classified by the underlying morphology and can be subdivided as injuries to the skull itself and intracranial injuries, which can be focal or diffuse (see Table 50B.1).
Skull Fractures
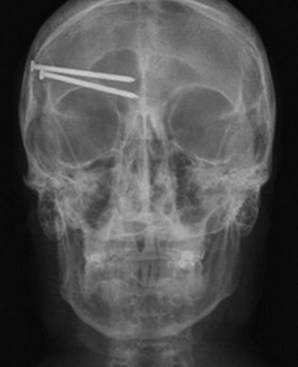
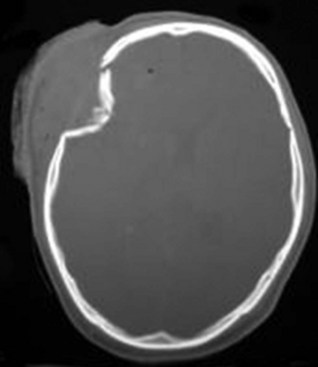
There are two types of skull fractures. Linear fractures traverse the full thickness of the skull from the outer to inner table, are usually fairly straight, and involve no bone displacement. The common method of injury is blunt force trauma in which the energy from the blow is transferred over a wide surface area of the skull. Fractures can be located in the calvarium or in the skull base. Skull base fractures are associated with cranial nerve (CN) palsy. Depending on their location, CN VII (temporal bone) and CN VI (clivus) are at risk; they are also prone to causing cerebrospinal fluid (CSF) leaks (Fig. 50B.3). In depressed skull fractures, the method of injury is similar to linear skull fractures, but in this case the inner and outer table are more shattered, and pieces are displaced into the brain (Fig. 50B.4). They may or may not lacerate the dura. The presence of skull fractures consistently has been associated with a higher incidence of intracranial lesions, neurological deficits, and poorer outcome (Bullock et al., 2006a). Hence, cranial fractures are important indicators of intracranial injuries, and CT scanning should be performed to rule out serious intracranial pathology in all patients with a known or suspected cranial fracture. A large study of patients with a TBI found that 71% of 850 patients with a cranial fracture had an intracranial lesion (e.g., contusion, hematoma), compared with only 46% of 533 patients without a cranial fracture (Macpherson et al., 1990).
Intracranial Injuries
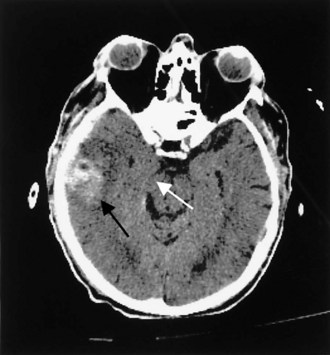
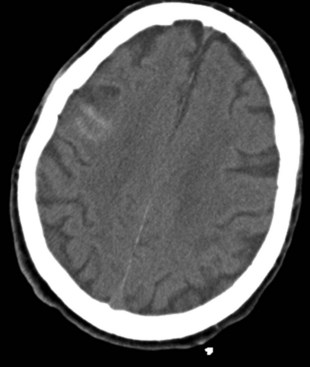
Contusions occur when the brain moves within the skull enough to collide with the skull, causing bruising of the brain parenchyma (hemorrhage and edema). The most common locations are the frontal and temporal poles, because these areas most often strike the bony surfaces of the skull base. Often, a second injury (contrecoup) is seen diagonally across the site of the direct impact (coup injury) when the accelerated brain strikes the skull on the opposite site. In contrast to concussion, contusions are visible on a head CT scan. Edema surrounding a contusion has lower signal intensity than brain on CT scan (Fig. 50B.5). Hemorrhages have higher CT signal intensity and are commonly seen as multiple bright areas of variable size. Progression of contusions is common, with delayed hemorrhage occurring over the first 24 hours in 25% of cases. However, neurosurgical intervention is rare.
An epidural hematoma (EDH) is bleeding between the dura and the skull. The peak incidence of EDH occurs in the second decade, and the mean age of TBI victims with EDH is between 20 and 30 years of age. Traffic-related accidents, falls, and assaults account for 53% of EDH in adults; falls account for half of EDH in children. Most (70%–90%) EDH are associated with a skull fracture and bleeding from a lacerated artery or indirect bone bleeding. Arterial bleeding was identified as the source of EDH in 36% of adults in a systematic review. Rupture of the middle meningeal vein, diploic veins, or venous sinuses also can cause EDH. The most common location of EDH is temporal; because the temporal bone is thinner than the rest of the skull, it breaks more easily, often resulting in a laceration of the middle meningeal artery (Bullock et al., 2006b) (Fig. 50B.6).
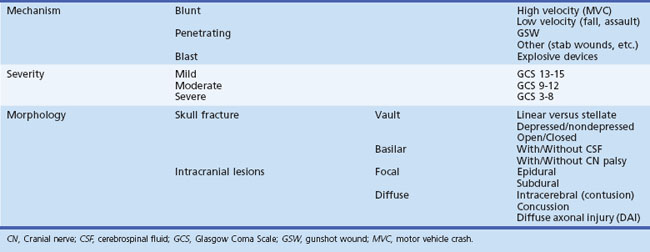
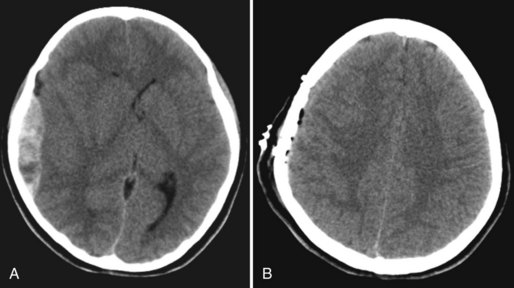
Subdural hematoma (SDH) develops between the brain parenchyma and the dura (Fig. 50B.7). It occurs when the brain moves within the skull enough to tear a surface vein, also called a bridging vein, that runs from the brain surface to the dural venous sinus. The most common locations are the frontal and parietal convexities. On a CT scan, an acute SDH typically appears as a bright (hyperdense), extraaxial, crescent-shaped homogeneous collection of fluid that conforms to the cerebral surface. Unlike an EDH, its spread is not limited by suture lines; it can spread over the whole convexity, but it almost never crosses the midline. An SDH can be acute or chronic. Herein, acute SDH is defined as an SDH diagnosed within 14 days of TBI. Chronic SDH contain blood older than 14 days or blood of different ages. This accounts for their appearance. They may be of mixed density or isodense to gray matter. In these cases, it can be identified by its mass effects, including sulcal effacement, inward buckling of the gray/white interface, and presence of midline shift.
In studies conducted after the introduction of CT, the incidence of SDH was estimated to be 11% of all patients with TBI (Servadei et al., 2000). The most common injuries involving traumatic SDH are motor vehicle collisions in the younger population and falls in those older than 75 years. Between 37% and 80% of patients with acute SDH present with an initial GCS score of 8 or less, and dilated pupils are seen preoperatively in 50% of these patients. Mortality among patients who arrive at the hospital in a coma and undergo surgical evacuation is between 57% and 68%.
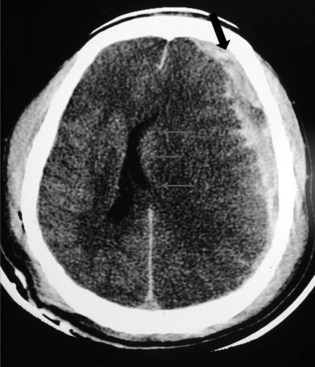
Traumatic subarachnoid hemorrhage (tSAH), seen with mild and severe TBI alike, refers to microhemorrhages into the subarachnoid space from crushed or ruptured smaller vessels. CT scans show hyperdense linear areas in superficial brain sulci (Fig. 50B.8). In contrast to aneurysmal SAH, the blood is superficial in the cortex and not present in the basal cisterns. In some instances, for example, if blood is found in the sylvian fissure, a vascular study (CT angiography, four-vessel digital subtraction angiography) is needed to rule out an aneurysm rupture. Most of the time, traumatic SAH indicates a more severe underlying brain injury and is seen in conjunction with DAI, SDH, or EDH. It has been shown that the presence of traumatic SAH is an independent factor for poor outcome. This finding could reflect a more extensive underlying injury that CT scan or conventional MRI cannot detect, or it could be attributable to a less recognized phenomenon: posttraumatic vasospasm. The incidence of posttraumatic vasospasm in patients with a mean GCS score of 7 (range 3-15) is similar to that following aneurysmal SAH (see Chapter 51C). Posttraumatic vasospasm is often overlooked, but one study found that hemodynamically significant vasospasm has been found in 44% of patients with tSAH (Oertel et al., 2005). Patients were at highest risk of developing hemodynamically significant vasospasm on day 3. Younger patients and patients with GCS scores of 8 or less were more likely to develop posttraumatic vasospasm.
Diffuse axonal injury is very common severe head injuries and is associated with significant morbidity. It is characterized clinically by rapid progression to coma in the absence of specific focal lesions. Adam classified DAI as mild, moderate, or severe (Adams et al., 1989). The duration of coma after a TBI correlates with the severity of DAI. Patients who survive the most severe form rapidly lapse into coma and remain unconscious, vegetative, or severely disabled.
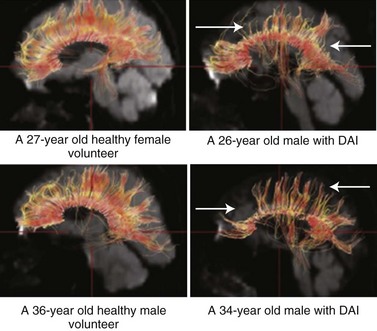
MRI is more sensitive than CT in detecting DAI. Gradient echo MRI is most sensitive to areas of hemorrhage, and fluid-attenuated inversion recovery images are best for visualizing nonhemorrhagic lesions. Diffusion tensor imaging is a new imaging technology more sensitive to DAI than conventional MRI. With DTI, the connectivity and integrity of neuronal tracts can be investigated. DTI is helpful when imaging tissue with an internal structure such as neural axons in white matter of the brain. Water molecules will then diffuse more rapidly in the direction aligned with the internal structure, and more slowly as it moves perpendicular to the preferred direction. With this information, injury to the white matter (diffusion anisotropy) can be detected and neural tracts followed through the brain (Fig. 50B.9). DTI measurements correlate with injury severity (acute GCS score) and outcome (Rankin Score at discharge) (Li and Feng, 2009; Sugiyama et al., 2007).
Pathophysiology
Following primary brain injury, a cascade of cellular and biochemical events occur in the initial minutes and extend into the subsequent days, leading to secondary neuronal degeneration (secondary brain injury) and ultimately neuronal impairment or cell death. Much of the research concerning TBI is directed at affecting the mechanisms of secondary brain injury. These mechanisms include free radical species, excitatory amino acid toxicity, hypoxia, impaired cerebrovascular autoregulation, hyperthermia, blood-brain barrier breakdown, and hypercalcemia. Hypoxia and hypoperfusion are recognized as leading contributors to secondary brain injury. A single hypotensive episode in which systolic blood pressure falls below 90 mm Hg has been associated with worse outcomes after severe TBI (Chesnut et al., 1993a). All these factors contribute to brain swelling, which leads to increased ICP, further exacerbating secondary injury.
Cerebrovascular dysregulation, often seen after TBI, is thought to contribute to secondary brain ischemia. One study found that cerebral blood flow (CBF) that was decreased (<18 mL/100 g/min) in the initial 4 to 12 hours after injury was associated with poor outcome (Piek et al., 1992). Increased CSF levels of endothelin-1 also are implicated in cerebrovascular dysregulation. Elevated CBF is often seen, especially in children and younger trauma patients. An increase in cerebral perfusion pressure (CPP) can be detrimental for such patients. Multimodal monitoring with a CBF probe, cerebral oximetry, or transcranial Doppler can help identify these patients so that elevated ICP can be treated appropriately.
After a TBI, excitotoxicity occurs upon the release of excessive amounts of excitatory amino acids such as glutamate, resulting in neuronal injury. Excitotoxic damage occurs in two phases: sodium-dependent neuronal swelling, followed by delayed calcium-dependent neuronal degeneration. These effects are mediated through activation of N-methyl-d-aspartate (NMDA) and glutamate receptors, leading to a rise in the intracellular calcium-mediated activation of proteases and lipases, which facilitates neuronal degeneration and necrotic cell death. In contrast to necrotic cell death, apoptosis or programmed cell death is marked not by swelling and dissolution of cell membranes but rather by DNA fragmentation and the formation of apoptotic cell bodies associated with neuronal shrinkage. Apoptosis is a cellular event triggered by either intrinsic mechanisms (initiated in the mitochondria) or extrinsic mechanisms (tumor necrosis factor [TNF] superfamily of cell-surface death receptors) which activate a cascade of enzymes called caspases that lead to cell termination. Apoptosis is thought to contribute to secondary neuronal injury after a TBI event. Animal studies have shown that developing neurons are more susceptible than mature neurons to excitotoxic injury, probably because more calcium is transmitted via the NMDA-mediated calcium channel in the immature brain (Geddes-Klein et al., 2006). However, although the administration of NMDA antagonists following TBI in immature rats led to decreased excitotoxic-mediated neuronal death, apoptotic cell death increased. The role of excitotoxicity and apoptosis following trauma to the developing brain warrants further investigation.
Studies of CSF support a role for inflammation after TBI. Interleukin (IL)-6, IL-8 (Minambres et al., 2003; Gopcevic et al., 2007), and soluble adhesion molecules (i.e., sICAM-1 [Otto et al., 2000]) were increased in the CSF following TBI. Whether these inflammatory mediators contribute to neurodegeneration or neuroprotection remains to be determined.
Treatment
Evaluation
Early intervention is essential in successful TBI care; as in any emergency situation, the CABs (circulation, airway, breathing) apply. Another priority of prehospital care is to optimize perfusion and oxygenation to prevent hypoxia and hypertension. Once in the emergency room, further measures can be taken if necessary to achieve adequate cardiopulmonary function. Next, a neurological examination is performed to triage patients accordingly. The GCS score is widely used to convey the severity of TBI, but a low GCS score upon arrival in the emergency room may be the result of sedation in the field. Only after the patient is weaned from sedation can injury severity be accurately assessed. Trauma patients with altered mental status, pupillary asymmetry, and flexion or extension posturing are at high risk for SDH or EDH compressing the brain and brainstem and must be evaluated with a CT scan of the head. CT is central to acute TBI diagnosis. There are two externally validated rules for when to require a CT scan after mild TBI: the Canadian CT Head Rule (CCTHR) (Stiell et al., 2001) and the New Orleans Criteria (NOC) (Haydel et al., 2000). These rules have 100% sensitivity for neurosurgical lesions and 83% to 98% sensitivity for nonoperative lesions. The CCTHR has a greater specificity and hence has led to fewer CT scans than the NOC (Levine, 2010). Neither addresses the presence of coagulopathy and anticoagulation, which others deem important risk factors for intracranial hemorrhage (Cohen et al., 2006; Ivascu et al., 2008). These criteria were updated in 2007 by the World Health Organization Taskforce on mild TBI and the Neurotraumatology Committee of the World Federation of Neurosurgical Societies (Fabbri et al., 2004) and in 2008 by the Centers for Disease Control and Prevention and the American College of Emergency Physicians. All in all, research findings agree on several indications for urgent CT scanning of the head after a minor TBI (see Table 50B.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree